Calorimetria - Aula 01 (Calor sensível)
Summary
TLDRIn this educational video, Professor Davi Oliveira introduces the concepts of thermal energy and heat, focusing on key topics like temperature, energy transfer, and specific heat. He explains how heat is transferred between bodies with different temperatures and covers the concept of sensible heat—heat that causes a change in temperature. The video includes practical examples, like heating water and cooling drinks, to demonstrate how sensible heat works. Additionally, the professor touches on latent heat and introduces the fundamental equations in calorimetry, providing an accessible explanation for students to understand these thermal principles in physics.
Takeaways
- 😀 Energy thermal energy is the sum of the energies of agitation of particles in a body, depending on its temperature and the number of molecules present.
- 😀 Heat is the transfer of thermal energy between bodies with different temperatures. The body with a higher temperature will transfer heat to the body with a lower temperature.
- 😀 Thermal equilibrium is reached when two objects in contact with different temperatures eventually attain the same temperature.
- 😀 Heat is constantly exchanged between our bodies and the environment, whether we are gaining or losing heat throughout the day.
- 😀 Sensible heat refers to the type of heat that causes a change in temperature, either increasing or decreasing it, but not changing the state of the substance.
- 😀 An example of sensible heat: when water’s temperature increases from 10°C to 30°C, it has gained sensible heat from the fire.
- 😀 Calorimetry uses the formula Q = mcΔT to calculate the amount of heat gained or lost, where Q is the quantity of heat, m is the mass, c is the specific heat capacity, and ΔT is the temperature change.
- 😀 Specific heat is the amount of heat needed to raise the temperature of 1 gram of a substance by 1°C. Each substance has a unique specific heat value.
- 😀 The SI unit of heat is joules, but calories are often used in educational contexts for simplicity, where 1 calorie equals approximately 4.2 joules.
- 😀 The concept of latent heat was introduced, which causes a change in the state of matter (e.g., fusion, condensation), but it does not affect the temperature of the substance during the phase change.
Q & A
What is thermal energy?
-Thermal energy is the sum of the kinetic energies of the particles in a body. It depends on the temperature and the number of molecules present in the system.
How does thermal energy transfer between bodies with different temperatures?
-Thermal energy transfers from the body with higher temperature to the one with lower temperature until both reach thermal equilibrium.
What is the concept of 'heat' in thermodynamics?
-Heat is thermal energy in transit. It refers to the transfer of thermal energy from one body to another due to a temperature difference.
What is the difference between heat and cold in terms of energy transfer?
-Cold is the sensation felt when the body loses heat to the environment. In contrast, heat is the energy that the body gains from the environment.
What is the definition of sensible heat?
-Sensible heat is the amount of heat absorbed or released by a body that results in a temperature change. It is associated with a change in temperature.
What happens to the temperature during a phase change?
-During a phase change (e.g., melting, boiling, or condensation), the temperature of a substance remains constant even though heat is being absorbed or released.
How is sensible heat calculated?
-Sensible heat is calculated using the formula: Q = mcΔT, where Q is the heat (in calories or joules), m is the mass of the substance, c is the specific heat capacity, and ΔT is the temperature change.
What is the relationship between calories and joules?
-One calorie is approximately equal to 4.2 joules. This conversion is important when switching between different units of energy in thermodynamics.
How do you calculate the specific heat of a material using calorimetry?
-The specific heat of a material can be determined using the formula: Q = mcΔT. If the heat (Q), mass (m), and temperature change (ΔT) are known, the specific heat (c) can be solved by rearranging the formula.
Why do different substances have different specific heats?
-Different substances have different specific heats because the amount of heat required to raise the temperature of 1 gram of a substance by 1°C depends on its molecular structure and properties.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes

Termologia - Conceitos Iniciais - Aula 01
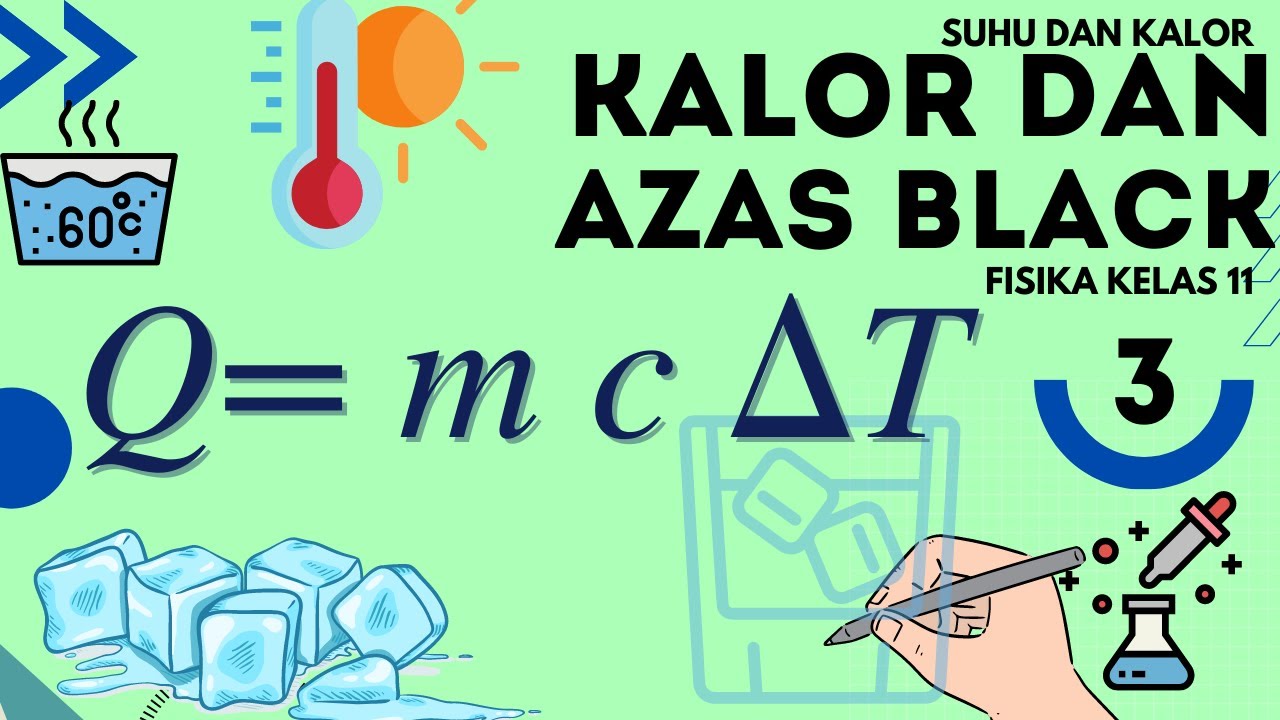
Suhu dan Kalor Fisika Kelas 11 - Part 3 : Kalor dan Azas Black

1ª LEI DA TERMODINÂMICA | Resumo de Física para o Enem

KALOR DAN PERPINDAHAN KALOR SERTA CONTOH SOAL DAN PENYELESAIANNYA|| Kelas XI SMA || Gasal

Calor sensível e calor latente conceitos para entender definitivamente

Heat and Temperature
5.0 / 5 (0 votes)
