Animasi Entalpi Molar - Termokimia Part 5 🔥🌡
Summary
TLDRThis video explains the concept of thermochemical equations, which describe the energy changes accompanying chemical reactions. It covers topics such as enthalpy, the relationship between reactant amounts and energy released, and standard conditions for measuring energy changes (25°C, 1 ATM). The script elaborates on specific types of enthalpy, including combustion enthalpy, formation enthalpy, and decomposition enthalpy, with examples involving hydrogen and carbon compounds. The video concludes with an invitation to subscribe, like, share, and access downloadable content, emphasizing the importance of understanding thermochemical processes in chemistry.
Takeaways
- 😀 A thermochemical equation shows the energy associated with a chemical reaction.
- 😀 Thermochemical equations include the enthalpy change (ΔH), which indicates the energy released or absorbed during the reaction.
- 😀 For example, the combustion of hydrogen with oxygen results in water and releases 286 kJ of energy.
- 😀 The energy produced or absorbed is proportional to the amount of substance involved in the reaction, e.g., 2 moles of hydrogen produce 572 kJ of energy.
- 😀 Enthalpy is often represented in kJ/mol, which indicates the amount of energy per mole of substance.
- 😀 Thermochemical data is typically measured at 25°C and 1 ATM pressure, which are considered standard conditions.
- 😀 Enthalpy changes are marked as ΔH° when measured at standard conditions and as ΔH otherwise.
- 😀 Standard enthalpy of combustion refers to the energy released when 1 mole of a substance is burned in oxygen under standard conditions.
- 😀 The standard enthalpy of formation refers to the energy change when 1 mole of a substance forms from its elements in their stable states.
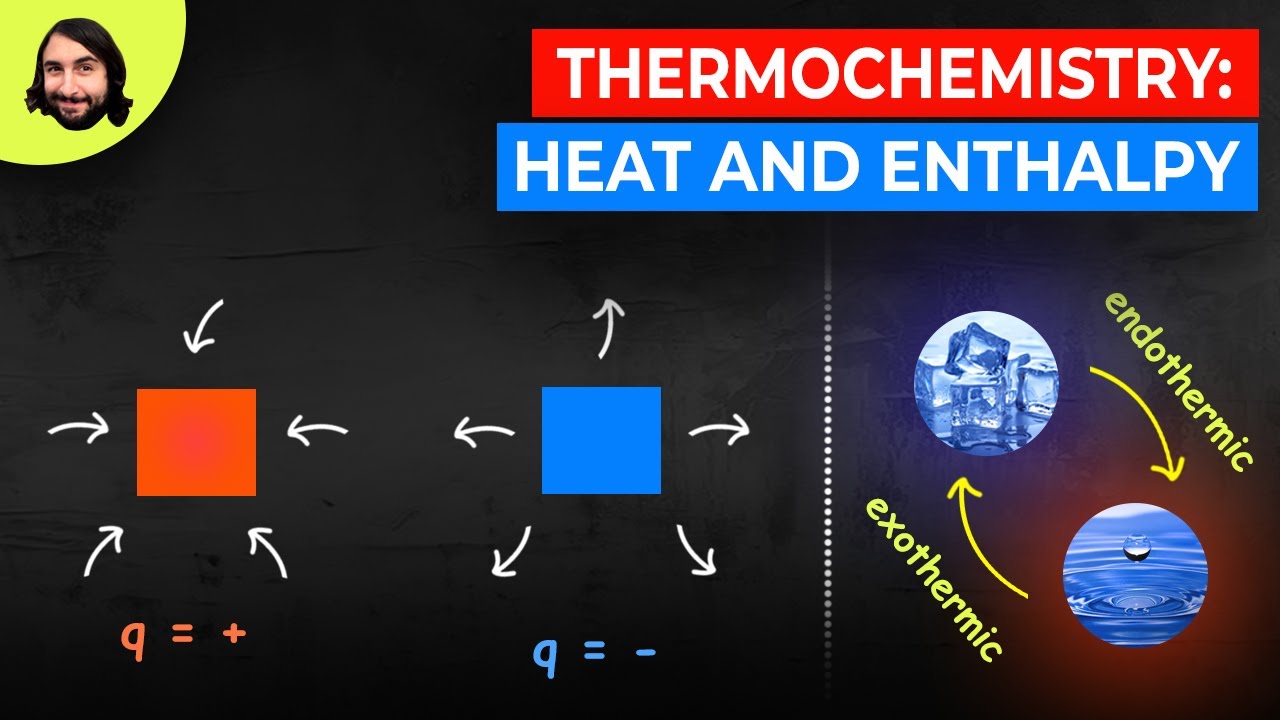
- 😀 A negative enthalpy change indicates that energy is released to the surroundings, while a positive change means energy is absorbed from the surroundings.
- 😀 Reactions discussed include combustion, formation, and decomposition reactions, each with specific enthalpy values.
Q & A
What is a thermochemical equation?
-A thermochemical equation is a chemical equation that shows the energy associated with a reaction, specifically the enthalpy change that accompanies the reaction.
How is enthalpy related to the amount of reactant in a chemical reaction?
-Enthalpy is directly proportional to the amount of substance reacting. For example, burning 1 mole of hydrogen gas produces 286 kJ of energy, while burning 2 moles would produce 572 kJ.
What does the symbol 'Delta H' represent in thermochemical equations?
-'Delta H' represents the change in enthalpy or the heat energy absorbed or released during a reaction.
What is the significance of enthalpy being expressed in kJ/mol?
-Enthalpy is often expressed in kilojoules per mole (kJ/mol) to indicate the amount of energy released or absorbed per mole of a substance during a chemical reaction.
What conditions are typically used to measure enthalpy values?
-Enthalpy values are typically measured at standard conditions: 25°C and 1 atm pressure. Under these conditions, enthalpy changes are denoted as 'Delta H°'.
What does 'Delta H' without the degree symbol (°) indicate?
-When 'Delta H' does not have the degree symbol (°), it indicates that the enthalpy change is measured under non-standard conditions, which could differ from the typical 25°C and 1 atm pressure.
What is enthalpy of combustion?
-Enthalpy of combustion is the enthalpy change when one mole of a substance undergoes complete combustion with oxygen at standard conditions, releasing energy.
What is enthalpy of formation?
-Enthalpy of formation is the enthalpy change when one mole of a compound is formed from its elements in their standard states.
What is enthalpy of decomposition?
-Enthalpy of decomposition is the enthalpy change when one mole of a compound breaks down into its constituent elements under standard conditions.
What does a negative sign in front of 'Delta H' signify?
-A negative sign in front of 'Delta H' indicates that energy is released by the system to the surroundings, such as in exothermic reactions.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes

Termokimia Bagian 1

TERMOKIMIA part 2- jenis-jenis perubahan entalpi standar Kimia kelas 11 semester 1

PERSAMAAN TERMOKIMIA | CARA MENULIS PERSAMAAN TERMOKIMIA | PAK BOSS Channel | Cerdas Kimia |

Termokimia: Kalor dan Entalpi | Kimia | SMA
Thermochemistry: Heat and Enthalpy

Termokimia • Part 2: Persamaan Termokimia dan Entalpi Molar
5.0 / 5 (0 votes)
