PERUBAHAN FISIKA DAN PERUBAHAN KIMIA | SIFAT DAN PERUBAHAN ZAT
Summary
TLDRThis educational video delves into the concept of material changes, distinguishing between physical and chemical properties. It explains that physical properties, such as shape, color, and density, do not alter the substance's composition, as seen in the melting of ice which remains H2O. In contrast, chemical changes result in new substances with different properties, exemplified by the burning of wood that transforms cellulose fibers into carbon. The video also highlights indicators of chemical changes, including the formation of new substances, gases, precipitates, color changes, and temperature shifts, aiming to deepen the viewer's understanding of these fundamental scientific principles.
Takeaways
- 🔍 The video discusses the concept of changes that objects undergo without any direct action being taken, such as food rotting or iron rusting.
- 📚 It emphasizes the importance of understanding the physical and chemical properties of substances to differentiate between the types of changes they undergo.
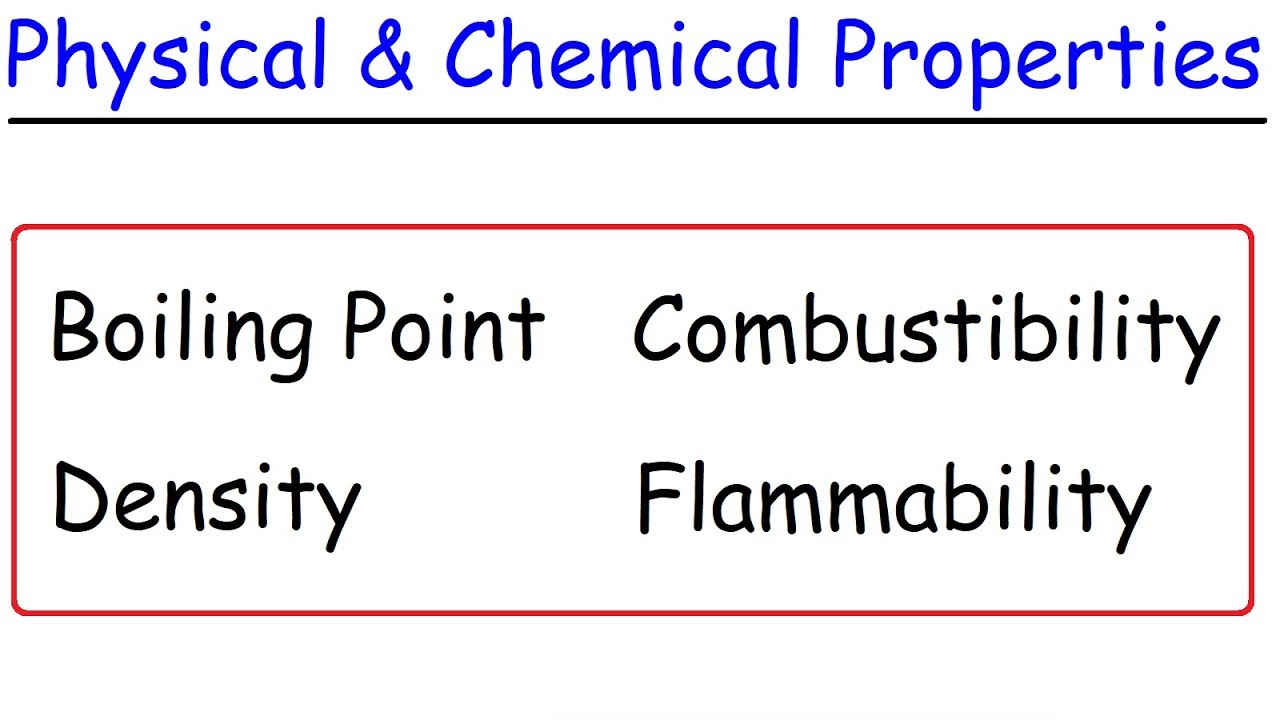
- 🌡️ Physical properties are those related to the physical state of a substance, including shape, color, smell, hardness, boiling point, freezing point, melting point, and density.
- 🔬 Chemical properties are associated with a substance's reactivity, including its ability to burn, rot, explode, rust, and its toxicity.
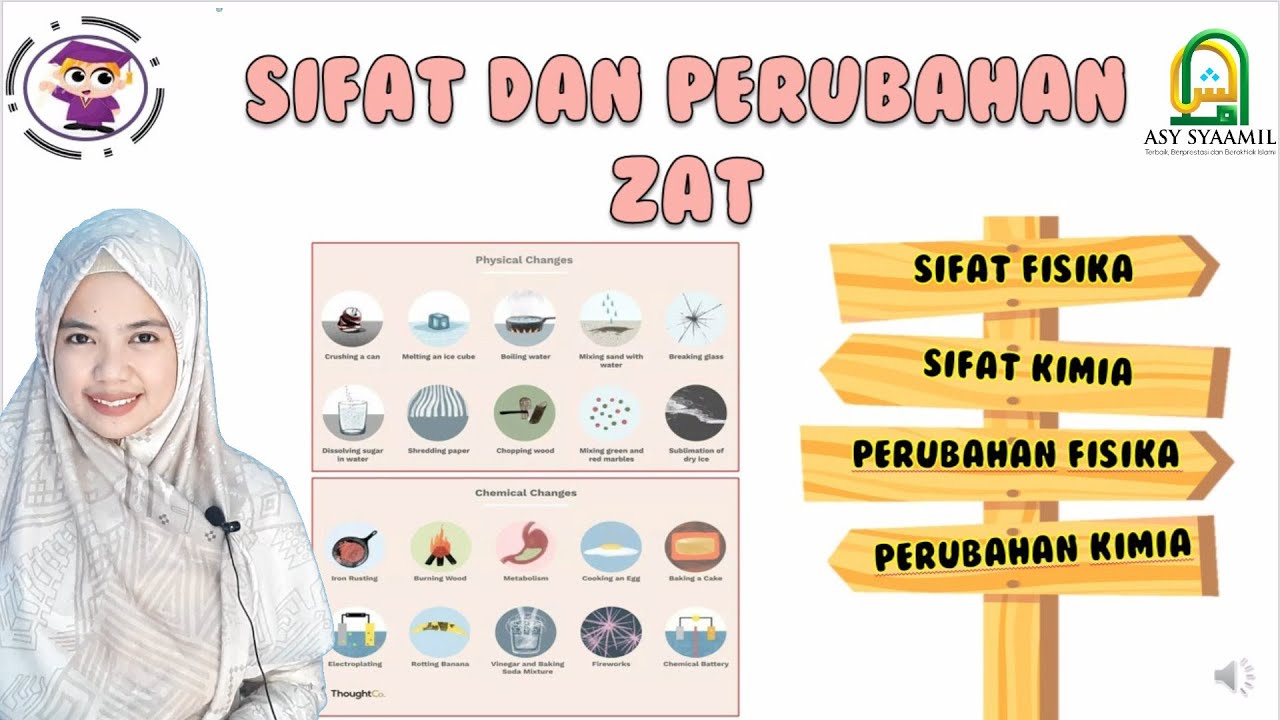
- 🔄 The script differentiates between physical changes, which do not result in the formation of new substances, and chemical changes, which do.
- 🧊 An example of a physical change is the melting of ice, which remains chemically H2O regardless of its state.
- 🔥 The burning of wood is given as an example of a chemical change, resulting in the formation of ash or carbon, which has different properties from the original wood.
- 🌲 The rusting of iron is another common example of a chemical change in nature, where a new substance with different properties is formed.
- 📋 The video provides characteristics to identify a chemical change, including the formation of new substances, gases, precipitates, color changes, and temperature changes.
- 🚫 Physical changes, in contrast, do not involve the creation of new substances with different structures.
- 📝 The script encourages viewers to rewatch and understand the difference between physical and chemical changes, and to download a summary from the video description if needed.
- 👍 The video ends with a call to action for likes, shares, and subscriptions for more educational content.
Q & A
What is the main topic of the video?
-The main topic of the video is the discussion of changes that objects undergo, both physical and chemical, without any action being taken.
What are some examples of objects undergoing changes without any action?
-Examples include bread, mushrooms, food spoiling, and rusting iron.
What are the two types of properties that objects can have?
-Objects can have physical properties and chemical properties.
What are some examples of physical properties of a substance?
-Physical properties include shape, color, smell, hardness, melting point, boiling point, freezing point, sublimation point, electrical conductivity, particle size, and mass.
What are some examples of chemical properties of a substance?
-Chemical properties include the ease or difficulty of burning, rotting, exploding, rusting, and toxicity.
What is a physical change?
-A physical change is a change in the state of a substance that does not involve the formation of a new substance.
What is a chemical change?
-A chemical change is a change that results in the formation of a new substance with different chemical properties.
What is an example of a physical change mentioned in the video?
-An example of a physical change is the melting of ice, where it changes from solid to liquid but remains H2O.
What is an example of a chemical change mentioned in the video?
-An example of a chemical change is the burning of wood, which results in the formation of ash or carbon, a substance with different properties from the original wood.
What are the characteristics that indicate a chemical change has occurred?
-Characteristics indicating a chemical change include the formation of a new substance, the production of gas, the formation of a precipitate, a change in color, and a change in temperature.
How can one distinguish between a physical change and a chemical change?
-A physical change does not result in the formation of a new substance, whereas a chemical change does.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen
5.0 / 5 (0 votes)