Misturas de soluções de solutos diferentes com reação química -Aula 10 |#soluções | #youtubeedu v1.0
Summary
TLDRThis lesson focuses on chemical solutions, particularly the reactions between acids, bases, and salts, as well as neutralization processes. It covers how to calculate molarity, understand the behavior of ions in solutions, and solve problems related to the pH of solutions. The video explains how to balance chemical equations, determine the final solution's acidity or basicity, and calculate the concentration of salts formed during reactions. The lesson also includes practical examples, such as mixing hydrochloric acid with sodium hydroxide, and provides insights into handling molarity and volume in neutralization reactions.
Takeaways
- 😀 The video discusses the process of mixing solutions of different solutes and their chemical reactions, specifically acid-base reactions forming salt and water.
- 😀 An example of mixing HCl solution with NaOH solution is used to determine whether the final solution is acidic, basic, or neutral.
- 😀 To assess the pH of the final solution, the number of moles of H+ and OH- ions is calculated and compared to determine the excess ion.
- 😀 The calculation for molarity (moles of solute per volume of solution) is essential in determining the concentration of H+ and OH- ions.
- 😀 The solution becomes neutral if H+ and OH- ions are present in equal amounts, and if there is an excess of either ion, the solution is either acidic or basic.
- 😀 A case study using HNO3 and NaOH is analyzed, where the reaction forms sodium nitrate (NaNO3) and water.
- 😀 The molarity of the resulting solution of NaNO3 is determined by calculating the number of moles formed and dividing by the final volume.
- 😀 A reaction between sulfuric acid (H2SO4) and silver hydroxide (AgOH) is discussed, showing how a base-neutralization reaction leads to the formation of silver sulfate and water.
- 😀 The volume of base required for neutralization in specific acid-base reactions is calculated using the molarity and volume of acid and base solutions.
- 😀 The video also discusses the formation of salts like sodium chloride (NaCl) when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), illustrating the stoichiometry of acid-base reactions.
Q & A
What is the main focus of the lesson in this video?
-The lesson focuses on the concept of solutions, particularly how different solutes, like acids and bases, react in chemical reactions to form salts and water. It also discusses calculating molarity and understanding pH levels.
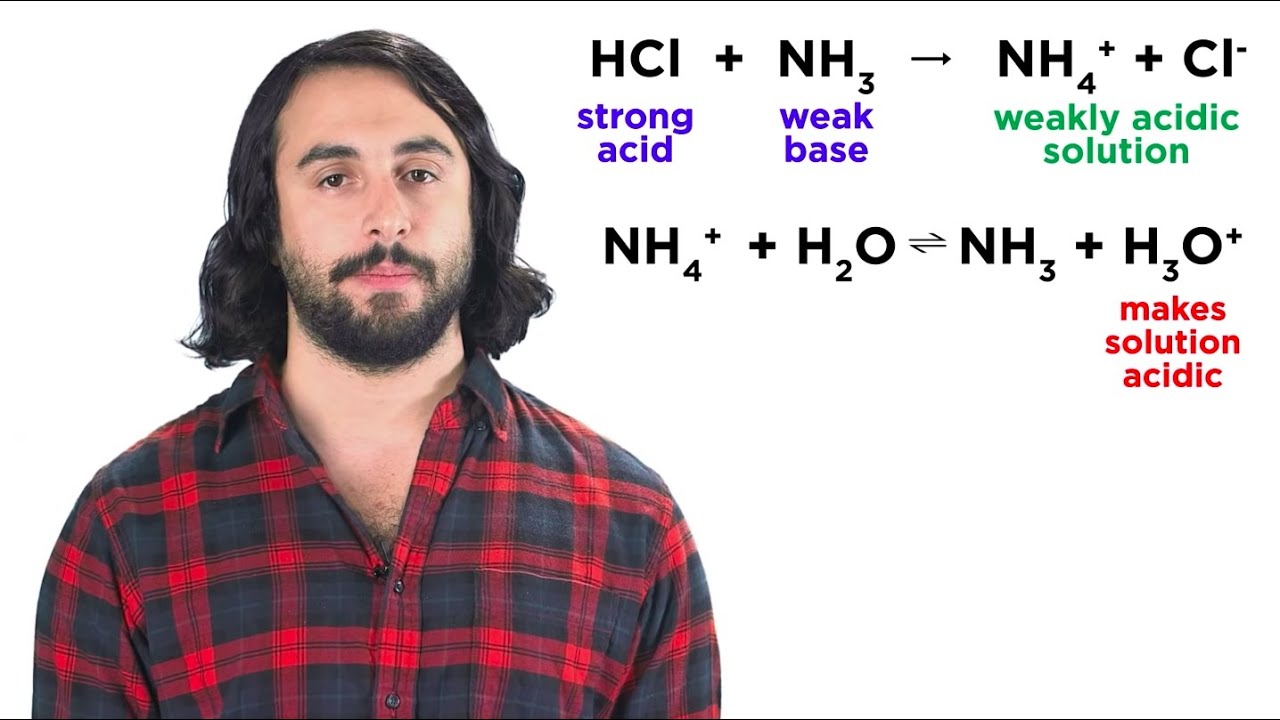
What happens when an acid like HCl reacts with a base like NaOH?
-When HCl reacts with NaOH, a neutralization reaction occurs, forming salt (NaCl) and water. The equation for this reaction is HCl + NaOH → NaCl + H2O.
How do you determine if a solution is acidic, basic, or neutral after mixing two solutions?
-To determine whether the solution is acidic, basic, or neutral, calculate the mols of H+ ions (acid) and OH- ions (base). If the quantities are equal, the solution is neutral. If there are more H+ ions, the solution is acidic, and if there are more OH- ions, the solution is basic.
How do you calculate the number of moles of H+ or OH- in a solution?
-To calculate the number of moles of H+ or OH-, multiply the molarity of the solution by its volume in liters. The formula is: moles = molarity × volume.
What does it mean when the reaction between an acid and base is 'one-to-one'?
-A 'one-to-one' reaction means that for every mole of H+ from the acid, one mole of OH- from the base will react, leading to the formation of one mole of water.
In the example of HCl and NaOH, what was the outcome of the reaction?
-In the example, HCl reacted with NaOH, resulting in the formation of NaCl (sodium chloride) and water. The final solution had an excess of OH- ions, making the solution basic.
What is the relationship between molarity and pH in a solution?
-The molarity of H+ ions in a solution is crucial in determining the pH. The pH is calculated as the negative logarithm of the H+ ion concentration (pH = -log[H+]). A lower pH indicates an acidic solution, while a higher pH indicates a basic solution.
How do you calculate the molarity of a solution when mixing two different solutions?
-To calculate the final molarity when mixing two solutions, first calculate the moles of each solute based on their respective volumes and molarities. Then, find the total volume of the mixed solution, and divide the total moles of solute by the total volume of the solution.
In the example with HNO3 and NaOH, how did the teacher determine the final pH?
-In the HNO3 and NaOH example, the teacher calculated the moles of H+ and OH- ions, determined the limiting reactant (the smaller quantity of ions), and found that after the neutralization, an excess of H+ ions remained, making the solution acidic with a final pH based on the remaining H+ concentration.
What is the significance of knowing the number of moles of ions in a solution?
-Knowing the number of moles of ions in a solution helps determine the solution's acidity or basicity, calculate pH, and determine the molarity of substances after a chemical reaction, ensuring proper neutralization and solution strength.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen

Sais: Definição e Classificações - Brasil Escola

Química Simples #54 - [Sais] - Conceito

Acids, Bases and Salts | Full Chapter | Class 10

ACIDS, BASES AND SALTS in 1 Shot FULL CHAPTER IN ANIMATION ||| NCERT SCIENCE Class 10th Chapter 2

IPA kelas 9 BAB 5 reaksi kimia dan dinamikanya kurikulum merdeka asam dan basa
Neutralization Reactions
5.0 / 5 (0 votes)
