Kesetimbangan kimia dalam industri (Nonuna)
Summary
TLDRIn this presentation, Kayla Latifah from the Nona group discusses the significance of chemical equilibrium in industrial processes. She explains the concept of chemical equilibrium, differentiating between static and dynamic types, and provides examples. The presentation explores the applications of equilibrium in industrial chemistry, focusing on the production of ammonia and sulfuric acid, highlighting how increasing temperature and using catalysts improve these processes. Kayla emphasizes the importance of understanding chemical equilibrium for efficient production of essential chemicals used in fertilizers, pharmaceuticals, and other industries, and closes with a thoughtful acknowledgment of the learning process.
Takeaways
- 😀 Chemical equilibrium refers to the state where the concentrations of reactants and products remain constant over time.
- 😀 Chemical equilibrium can only occur in reversible reactions, where reactants can form products and vice versa.
- 😀 Static equilibrium occurs in one-way reactions, where products cannot revert to reactants (e.g., burning paper).
- 😀 Dynamic equilibrium occurs in reversible reactions, where both forward and backward reactions occur simultaneously (e.g., water boiling and condensing).
- 😀 Homogeneous equilibrium involves reactions where all substances are in the same phase (e.g., ammonia formation).
- 😀 Heterogeneous equilibrium involves reactions with components in different phases (e.g., sulfur combustion).
- 😀 Increasing temperature and adding catalysts can speed up chemical reactions in industrial processes (e.g., ammonia production).
- 😀 Ammonia production at room temperature is slow, but heating it to 650°C and adding a catalyst accelerates the process.
- 😀 Sulfuric acid production shifted from the chamber process to the contact process for better efficiency and faster reactions.
- 😀 Chemical equilibrium plays a vital role in the production of essential industrial chemicals such as ammonia, nitric acid, and sulfuric acid.
- 😀 Chemicals like ammonia are used in various industries such as fertilizers, nylon production, pharmaceuticals, and detergents.
Q & A
What is chemical equilibrium?
-Chemical equilibrium is a state where the concentrations of reactants and products remain constant over time, meaning there is no net change in the system. It occurs in reversible reactions, where both the forward and reverse reactions happen at the same rate.
What is the difference between static and dynamic equilibrium?
-Static equilibrium occurs in reactions that proceed in only one direction, and once the reaction reaches completion, no further changes occur. Dynamic equilibrium, on the other hand, involves reactions that can proceed in both directions, with reactants and products continuously converting into each other at equal rates.
Can you provide an example of static equilibrium?
-An example of static equilibrium is the combustion of paper. Once paper is burned and turned to ash, it cannot revert to its original state.
What is an example of dynamic equilibrium?
-An example of dynamic equilibrium is the boiling of water. When steam condenses and turns back into water, both processes occur simultaneously, leading to a dynamic balance between liquid water and water vapor.
How is chemical equilibrium categorized based on the phases of substances involved?
-Chemical equilibrium can be categorized into two types based on the phases of the substances: homogeneous equilibrium, where all the substances involved are in the same phase, and heterogeneous equilibrium, where the substances are in different phases.
What is the difference between homogeneous and heterogeneous equilibrium?
-In homogeneous equilibrium, all components of the reaction are in the same phase, such as in the synthesis of ammonia. In heterogeneous equilibrium, components exist in more than one phase, like in the combustion of sulfur.
How does temperature affect the production of ammonia?
-Ammonia production is slow at room temperature, but by raising the temperature to 650°C, the rate of reaction increases, producing ammonia more quickly. This process is commonly used in industry to meet demand efficiently.
What role does a catalyst play in ammonia production?
-A catalyst, often used in ammonia production, speeds up the reaction rate without being consumed in the process. It helps increase the yield of ammonia by facilitating the reaction at the elevated temperatures.
Why is the contact process important in sulfuric acid production?
-The contact process is important because it allows for the efficient production of sulfuric acid by using high temperatures and a catalyst, such as platinum or vanadium. This process speeds up the reaction and prevents issues like corrosion in earlier methods.
How are industrial chemicals, like ammonia and sulfuric acid, related to chemical equilibrium?
-Chemical equilibrium is crucial in the production of industrial chemicals like ammonia and sulfuric acid because it helps determine the optimal conditions (temperature, pressure, catalysts) for maximizing the yield and efficiency of these reactions in manufacturing processes.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen
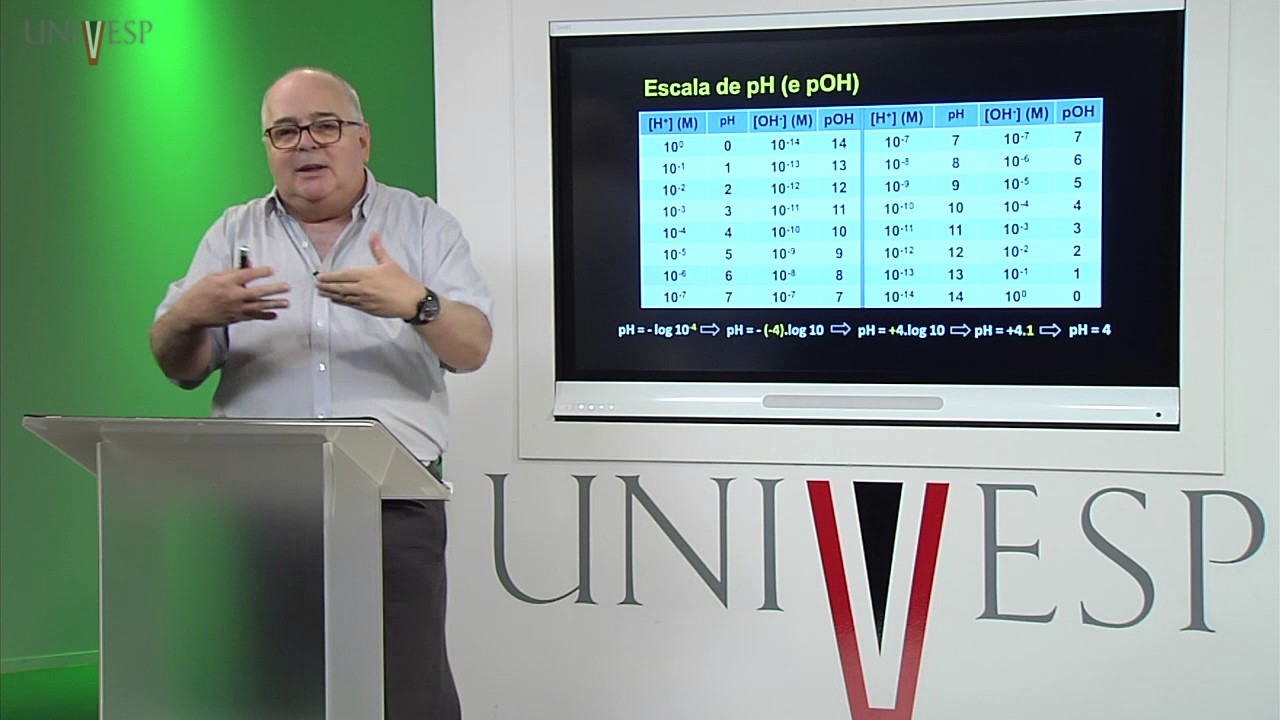
Bioquímica - Aula 03 - Alguns conceitos químicos importantes - 2

Faktor-Faktor yang Mempengaruhi Pergeseran Kesetimbangan dan Penerapannya dalam Industri

KESETIMBANGAN KIMIA KELAS 11_PART 1

VELOCIDAD DE REACCIÓN Y EQUILIBRIO QUÍMICO

Keseimbangan Pasar Barang menggunakan Kurva IS

Fisiologi Tanaman - Gerakan Partikel
5.0 / 5 (0 votes)
