UV-visible spectroscopy basics- Chromophore, auxochrome, spectral shifts
Summary
TLDRIn this video, the presenter delves into the fascinating world of spectroscopy, specifically focusing on chromophores and electronic transitions such as hypsochromic and bathochromic shifts. The discussion covers the role of functional groups in contributing to molecular color and how different electronic configurations affect color intensity. Examples like beta-carotene and nitrobenzene are used to explain these concepts. The video further explores how UV-visible spectroscopy reveals absorption patterns and the effect of molecular geometry and resonance on color perception. It is a detailed exploration for viewers interested in the science of light absorption and molecular structures.
Takeaways
- 😀 The script covers important terminology related to pharmaceutical sciences and spectroscopy, specifically focusing on terms like 'hypochromic shift' and 'chromophores'.
- 😀 'Chromophores' are groups within molecules responsible for color in compounds, especially their absorption in the UV-visible spectrum.
- 😀 The concept of 'hypochromic shift' refers to a reduction in the intensity of color observed in molecules.
- 😀 The script explains how certain functional groups, such as those in compounds like β-carotene, are responsible for color intensity in organic molecules.
- 😀 Different types of electronic transitions in molecules are discussed, including those that involve electrons in bonds and non-bonding orbitals.
- 😀 The script mentions the effects of UV and visible light on molecular structures, particularly on how functional groups influence light absorption.
- 😀 Examples of compounds like β-carotene and nitrobenzene are used to explain how changes in the molecular structure can impact the intensity and color observed.
- 😀 The concept of 'resonance' is introduced, emphasizing how it affects the absorption and emission of light in chemical compounds.
- 😀 The use of spectroscopy in analyzing molecular structure and behavior, particularly in identifying functional groups and their role in absorption spectra, is highlighted.
- 😀 The interaction of electromagnetic radiation with molecules, particularly how it leads to color changes and absorption peaks, is discussed throughout the script.
Q & A
What is the significance of 'chromophore' in spectroscopy?
-A chromophore is a group of atoms in a molecule that is responsible for its color, which is due to its ability to absorb light at specific wavelengths. Chromophores are essential in spectroscopic studies as they provide insight into the molecular structure and the electronic transitions within the compound.
What are 'hypsochromic' and 'bathochromic' shifts in UV-Visible spectroscopy?
-'Hypsochromic' shifts refer to the phenomenon where the absorption maximum of a compound shifts to a shorter wavelength (bluer shift), while 'bathochromic' shifts refer to a shift to a longer wavelength (redder shift). Both shifts are influenced by changes in the molecular environment or structure.
How does the presence of functional groups affect color in molecules?
-Functional groups with lone pairs of electrons or conjugated systems (like -OH, -NH2, -COOH, etc.) can influence the electronic structure of molecules, affecting their ability to absorb certain wavelengths of light. This results in visible color changes, especially in compounds like dyes and pigments.
What is the role of electronic transitions in color change?
-Electronic transitions occur when electrons in a molecule move from one energy level to another, typically when the molecule absorbs photons. These transitions are responsible for the color observed in a substance, as different transitions correspond to absorption in different parts of the electromagnetic spectrum.
Can 'chromophores' in a molecule change the intensity of color?
-Yes, chromophores can increase the intensity of color by extending conjugation or by incorporating electron-donating or withdrawing groups that alter the absorption spectrum. The more electrons a chromophore can share or accept, the greater the absorption and thus, the intensity of color.
What is the significance of the term 'electronic absorption' in spectroscopy?
-Electronic absorption refers to the absorption of photons by electrons in a molecule, causing them to jump to a higher energy state. This process is fundamental in UV-Visible spectroscopy as it determines the wavelengths of light absorbed by a molecule, which in turn indicates its structure and functional groups.
How does 'resonance' affect the color of a molecule?
-Resonance in molecules refers to the delocalization of electrons across multiple atoms or bonds. This delocalization can lower the energy required for electronic transitions, thus allowing the molecule to absorb light at longer wavelengths and resulting in a more intense or distinct color.
What is the difference between 'hypochromic' and 'hyperchromic' effects?
-The 'hypochromic' effect occurs when the intensity of absorption decreases, leading to a lighter color, whereas the 'hyperchromic' effect refers to an increase in the intensity of absorption, leading to a darker or more intense color. These effects are influenced by the molecular environment or structural changes in the molecule.
What is the relationship between conjugation and the color of organic compounds?
-Conjugation, which refers to the alternating single and double bonds in a molecule, allows electrons to move freely across the structure. This electron delocalization reduces the energy gap for electronic transitions, enabling the compound to absorb light in the visible region, which results in a visible color.
How does UV-Visible spectroscopy help in understanding molecular structures?
-UV-Visible spectroscopy helps identify the electronic structure of molecules by analyzing the wavelengths of light they absorb. This information can reveal the presence of specific functional groups, conjugation, and molecular geometry, providing valuable insight into the molecular composition and behavior.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen
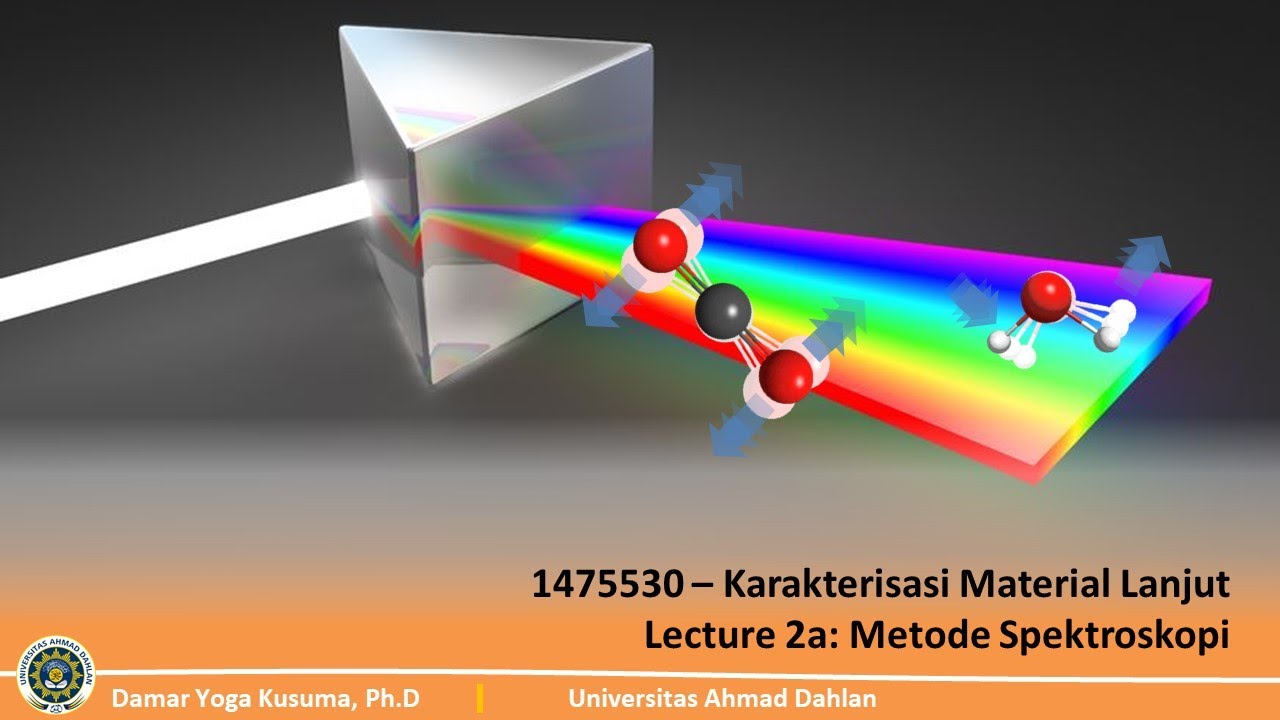
UAD - Kuliah Online 1475530 Karakterisasi Material Lanjut (Lecture 2a - part 1)

MK Prinsip Pengukuran Kimia - Spektroskopi Rotasi Murni

NMR.part4

Chemistry Class 12 | Chapter 12 | Topic 3b | UV-VIS Spectroscopy | in urdu | tutoria.pk

Retrovírus - Vírus - Biologia com o Tubarão
Ternyata Begini Jejak Manusia Purba PITHECANTHROPUS MOJOKERTENSIS Manusia Kera Dari Mojokerto
5.0 / 5 (0 votes)
