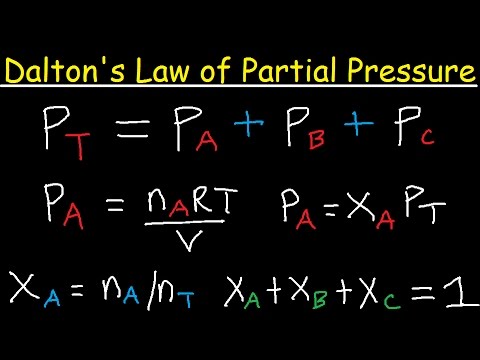
Fracción peso a fracción mol [PFP]
Summary
TLDRIn this video, the presenter explains how to convert weight fractions into mole fractions for a gas mixture. Using a step-by-step approach, the speaker covers essential concepts like weight fraction, mole fraction, and molecular weight. Through a detailed example, the audience learns how to calculate the number of moles for each gas component and then determine their mole fractions by dividing individual moles by the total moles in the system. This simple yet effective method is useful for accurately converting between these two types of fractions in various gas mixtures.
Takeaways
- 😀 Understand the relationship between weight fraction and mole fraction in a gas mixture.
- 😀 The weight fraction of a component can be used to calculate the mole fraction by using molecular weights.
- 😀 The number of moles of a component is calculated by dividing its mass by its molecular weight.
- 😀 To convert from weight fraction to mole fraction, follow a systematic procedure involving summing moles of each component.
- 😀 The mole fraction of each component is found by dividing its number of moles by the total number of moles in the system.
- 😀 The total number of moles in the system is the sum of moles from each component.
- 😀 The molecular weight of each component is essential for calculating the number of moles.
- 😀 Weight fractions of the components must add up to 1, ensuring consistency in the calculation.
- 😀 The process requires dividing each component's weight fraction by its molecular weight to obtain moles.
- 😀 A clear example is provided where the weight fractions and molecular weights of five components are used to demonstrate the conversion.
- 😀 The final mole fraction of each component is calculated by dividing the component's moles by the total moles in the system.
Q & A
What is the main purpose of the video?
-The video explains how to convert weight fractions to mole fractions in a mixture of gases in a simple and clear way.
What is the key equation mentioned for calculating mole fractions?
-The key equation is nj = wj / mj, where wj is the weight fraction of component j and mj is the molar mass of component j. The mole fraction of each component is then calculated using this formula.
What is the importance of the weight fraction in this process?
-The weight fraction represents the ratio of the mass of a specific component in the mixture to the total mass of the mixture. It is crucial for calculating the mole fraction.
How is the mole fraction of each component calculated?
-The mole fraction of each component is calculated by dividing the weight fraction of the component by the molar mass of that component and then summing the results of all components.
What do you do after calculating the mole fractions of each component?
-After calculating the mole fractions for each component, you ensure that the sum of these mole fractions equals 1. This confirms that the mole fraction calculations are correct.
What formula is used to calculate the number of moles of each component?
-The number of moles (n) of each component is calculated using the formula: n = mass / molar mass, where the mass is the weight fraction multiplied by the total mass and the molar mass is the molecular weight of the component.
Why is the sum of the weight fractions required to equal 1?
-The sum of the weight fractions must equal 1 because it represents the total mass of the mixture. This ensures that the weight fractions are properly normalized.
What is the relationship between the mole fraction and weight fraction?
-The mole fraction of a component is directly related to its weight fraction through its molar mass. The mole fraction is the weight fraction divided by the molar mass of the component, and then the mole fractions are summed to check that they equal 1.
What practical steps are taken in the example provided in the video?
-In the example, the weight fractions and molar masses of five components (methane, etc.) are used to calculate the mole fractions. The mole fractions are then computed by dividing the weight fractions by their respective molar masses.
How does following the correct order of steps ensure accuracy in calculations?
-Following the correct order of steps, such as calculating the mole fractions from the weight fractions, ensures that all components are properly accounted for. Skipping steps or doing them out of order could lead to inaccurate results.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen
5.0 / 5 (0 votes)