Química - Química Geral - Aula 33 - Fração Molar e Pressão Parcial dos Gases
Summary
TLDRThis video tutorial explains key concepts related to gas mixtures, such as mole fraction, partial pressure, and molar mass. The presenter uses the example of a balloon containing helium and hydrogen to demonstrate mole fractions and partial pressures. The video also covers real-world applications, such as the composition of the trimix gas used in diving, and how to calculate partial pressures and the average molar mass of a gas mixture. By the end of the video, viewers will understand how to calculate mole fractions, partial pressures, and molar masses in practical contexts.
Takeaways
- 😀 Fractions of gases in a mixture are calculated by dividing the amount of a specific gas (in moles) by the total amount of gas present in the container, known as the mole fraction.
- 😀 Mole fractions can be represented as percentages by multiplying the decimal value by 100.
- 😀 The mole fraction of helium in a mixture with 1 mole of helium and 3 moles of hydrogen is 0.25, while the mole fraction of hydrogen is 0.75.
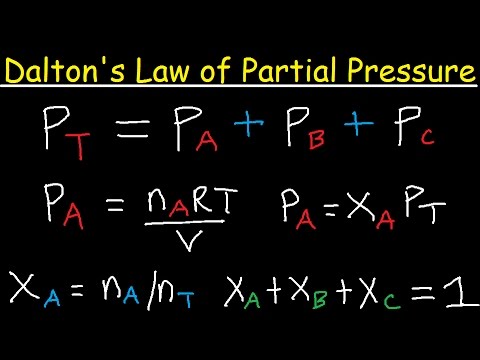
- 😀 The concept of **partial pressure** refers to the pressure that each gas would exert if it occupied the entire container by itself, despite being mixed with other gases.
- 😀 Partial pressure can be calculated using the formula: Partial Pressure = Mole Fraction × Total Pressure.
- 😀 For a mixture containing 1 mole of helium and 3 moles of hydrogen, the partial pressure of helium (0.25 mole fraction) is 0.25 atm, and the partial pressure of hydrogen (0.75 mole fraction) is 0.75 atm.
- 😀 An example of a gas mixture used in diving is **Trimix**, which consists of 16% oxygen, 24% helium, and 60% nitrogen by volume.
- 😀 To calculate the partial pressure of helium in the Trimix example, we multiply the helium's mole fraction (0.24) by the total pressure (9,000 kPa), resulting in a partial pressure of 2,160 kPa.
- 😀 To calculate the **average molar mass** of a gas mixture, each gas's contribution to the total mass is found by multiplying its mole fraction by its molar mass.
- 😀 The average molar mass of the Trimix gas mixture, with 16% oxygen, 24% helium, and 60% nitrogen, is 22.88 g/mol, based on their respective molar masses and mole fractions.
Q & A
What is the concept of molar fraction in gas mixtures?
-Molar fraction is the ratio of the amount of one particular gas to the total amount of all gases in a mixture. It is calculated by dividing the number of moles of a specific gas by the total number of moles of all gases in the system.
How do you calculate the molar fraction of helium in the example with 1 mole of helium and 3 moles of hydrogen?
-The molar fraction of helium is calculated as: (1 mol of helium) / (1 mol of helium + 3 mols of hydrogen) = 1 / 4 = 0.25 or 25%.
What does the molar fraction represent in terms of gas composition?
-The molar fraction represents the proportion of the total number of moles that a specific gas contributes to the mixture, which can also be expressed as a percentage.
What is the definition of partial pressure in the context of gas mixtures?
-Partial pressure is the pressure exerted by a single gas in a mixture, assuming the gas behaves independently. It can be calculated by multiplying the molar fraction of the gas by the total pressure of the mixture.
How do you calculate the partial pressure of helium in a mixture where the total pressure is 1 atm and the molar fraction of helium is 0.25?
-The partial pressure of helium is calculated by multiplying the molar fraction of helium (0.25) by the total pressure (1 atm): 0.25 x 1 atm = 0.25 atm.
How does the concept of partial pressure help in real-world applications, like diving with mixtures like Trimix?
-Partial pressure is crucial in applications like diving because it helps to determine the pressure exerted by each gas in the mixture at various depths, ensuring safe gas levels and preventing issues like nitrogen narcosis or oxygen toxicity.
What gases are present in the Trimix mixture used in diving, and in what proportions?
-Trimix consists of 16% oxygen (O₂), 24% helium (He), and 60% nitrogen (N₂), with the percentages representing the volume of each gas in the mixture.
How do you calculate the partial pressure of helium in a Trimix mixture with a total pressure of 9,000 kPa?
-The molar fraction of helium in Trimix is 24% (or 0.24). To calculate the partial pressure of helium, multiply the molar fraction by the total pressure: 0.24 x 9,000 kPa = 2,160 kPa.
What is the formula to calculate the average molar mass of a gas mixture, and how is it applied to Trimix?
-The average molar mass of a gas mixture is calculated by multiplying the molar fraction of each gas by its molar mass and then summing the results. For Trimix, this is calculated as: (0.16 x 32 g/mol for O₂) + (0.24 x 4 g/mol for He) + (0.60 x 28 g/mol for N₂) = 22.88 g/mol.
Why is it important to know the average molar mass of a gas mixture, especially in diving or medical applications?
-Knowing the average molar mass is important because it helps in understanding the behavior of the gas mixture, such as its density, which affects breathing resistance and the risks associated with different gas mixtures under pressure.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)