Phase diagrams | States of matter and intermolecular forces | Chemistry | Khan Academy
Summary
TLDRThis video explains phase changes under varying pressure conditions, focusing on water and carbon dioxide. It introduces phase diagrams, illustrating how temperature and pressure influence the transitions between solid, liquid, and gas. The script explores concepts such as freezing and boiling points, sublimation, and the critical point, where substances become supercritical fluids. Through examples like water at different altitudes and carbon dioxide in dry ice, the video emphasizes how changes in pressure and temperature can alter a substance's state, and the role of phase diagrams in predicting these behaviors.
Takeaways
- 😀 Pressure isn't always constant, and different pressure conditions affect phase changes.
- 😀 The phase diagram is a crucial tool for understanding the transition between states of matter (solid, liquid, gas) based on temperature and pressure.
- 😀 At 1 atmosphere of pressure, water transitions from solid to liquid at 0°C, and from liquid to gas at 100°C.
- 😀 Increasing pressure on water decreases the temperature at which ice melts, while lowering pressure increases the melting point.
- 😀 At higher altitudes, where pressure is lower, water freezes at higher temperatures and boils at lower temperatures.
- 😀 The transition from liquid to gas is influenced by air pressure, which prevents molecules from escaping into gas form under higher pressure.
- 😀 On the Moon or in a vacuum, a liquid can more easily turn into gas due to the lack of air pressure.
- 😀 Water can undergo sublimation at low pressures, transitioning from solid to gas directly, bypassing the liquid phase.
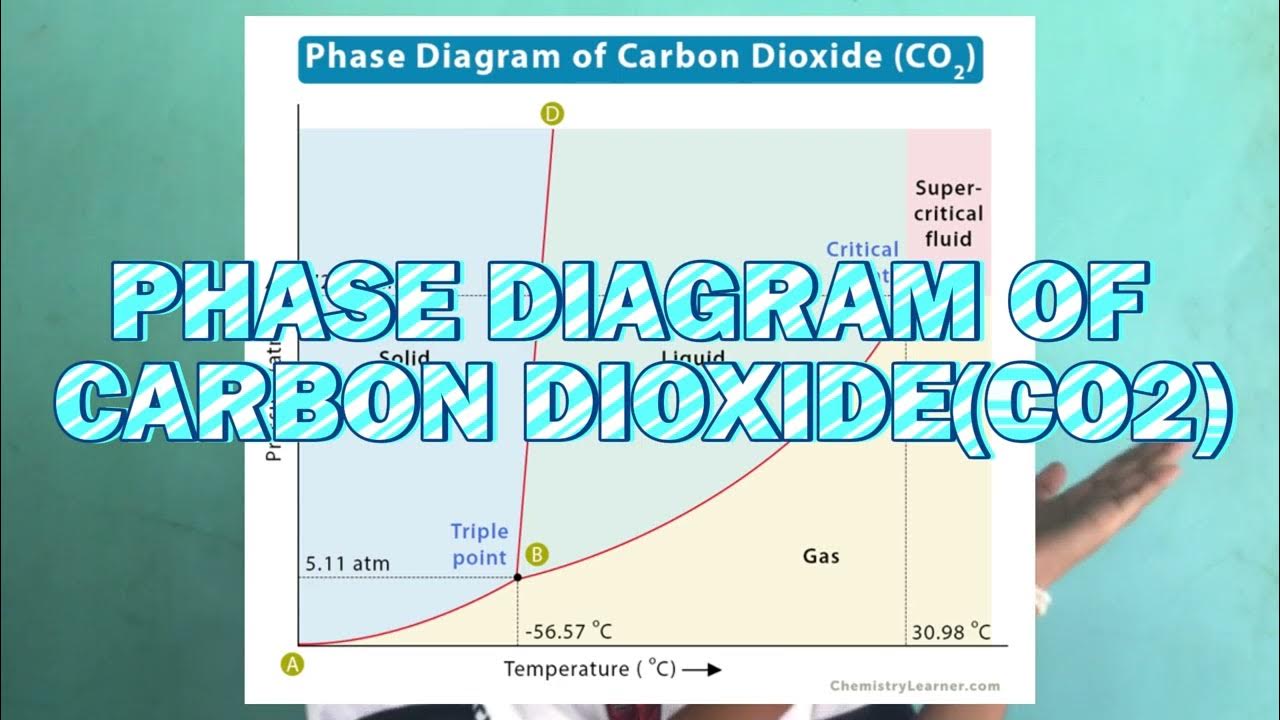
- 😀 Carbon dioxide has a different phase diagram than water, and at 1 atmosphere, it sublimates directly from solid (dry ice) to gas.
- 😀 A triple point is where a substance can coexist in all three phases—solid, liquid, and gas—at specific pressure and temperature conditions.
- 😀 The critical point represents a high-pressure and high-temperature state where the substance behaves as a supercritical fluid, having properties of both liquid and gas.
Q & A
What does a phase diagram show?
-A phase diagram illustrates the states of matter (solid, liquid, gas) and their transitions based on temperature and pressure.
How does pressure affect the freezing and melting points of water?
-Increasing pressure lowers the temperature at which ice melts, while decreasing pressure raises the freezing point.
What happens to the boiling point of water at higher altitudes like Denver or Mount Everest?
-At higher altitudes, where pressure is lower, the boiling point of water decreases, meaning water boils at a lower temperature.
What is the phase transition from solid to gas called, and under what conditions does it occur?
-The transition from solid to gas is called sublimation, which can occur at low pressures, such as on the moon or when dealing with dry ice at Earth’s standard pressure.
What is the role of air pressure in the evaporation or vaporization of liquids?
-Air pressure prevents molecules in a liquid from escaping into the gas phase. Lowering pressure makes it easier for molecules to escape and transition to gas.
What is the 'triple point' of a substance?
-The triple point is the unique temperature and pressure at which all three phases (solid, liquid, and gas) of a substance coexist in equilibrium.
What happens at the 'critical point' of a substance?
-At the critical point, a substance enters a supercritical fluid state, where it exhibits properties of both a liquid and a gas due to high temperature and pressure.
How does carbon dioxide behave differently from water in a phase diagram?
-Carbon dioxide sublimes directly from solid to gas at 1 atmosphere pressure and does not have a liquid phase at standard pressure on Earth, unlike water.
Why do substances like supercritical water have practical applications?
-Supercritical fluids, like supercritical water, are excellent solvents because they combine the properties of both liquids and gases, making them efficient for tasks like cleaning or dissolving substances.
What would happen if you increased the pressure on water at 100°C?
-Increasing the pressure on water at 100°C would prevent it from boiling, and it could transition to a liquid state instead, even at temperatures above its normal boiling point.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen
A PHASE DIAGRAM OF WATER (H2O) & CARBON DIOXIDE (CO2)

Phase Changes, Heats of Fusion and Vaporization, and Phase Diagrams

Phase Diagrams | Phase Diagram of Water and Phase Diagram of Carbon Dioxide

05. MG2112 Termodinamika Metalurgi (Segmen 04: Kesetimbangan Es-Air, Ice Skating)

HUBUNGAN PERHITUNGAN TEKANAN HIDROSTATIS TERHADAP DESAIN BENDUNGAN

EXPERIMENT PROTEIN DENATURED | ALPHA 6
5.0 / 5 (0 votes)
