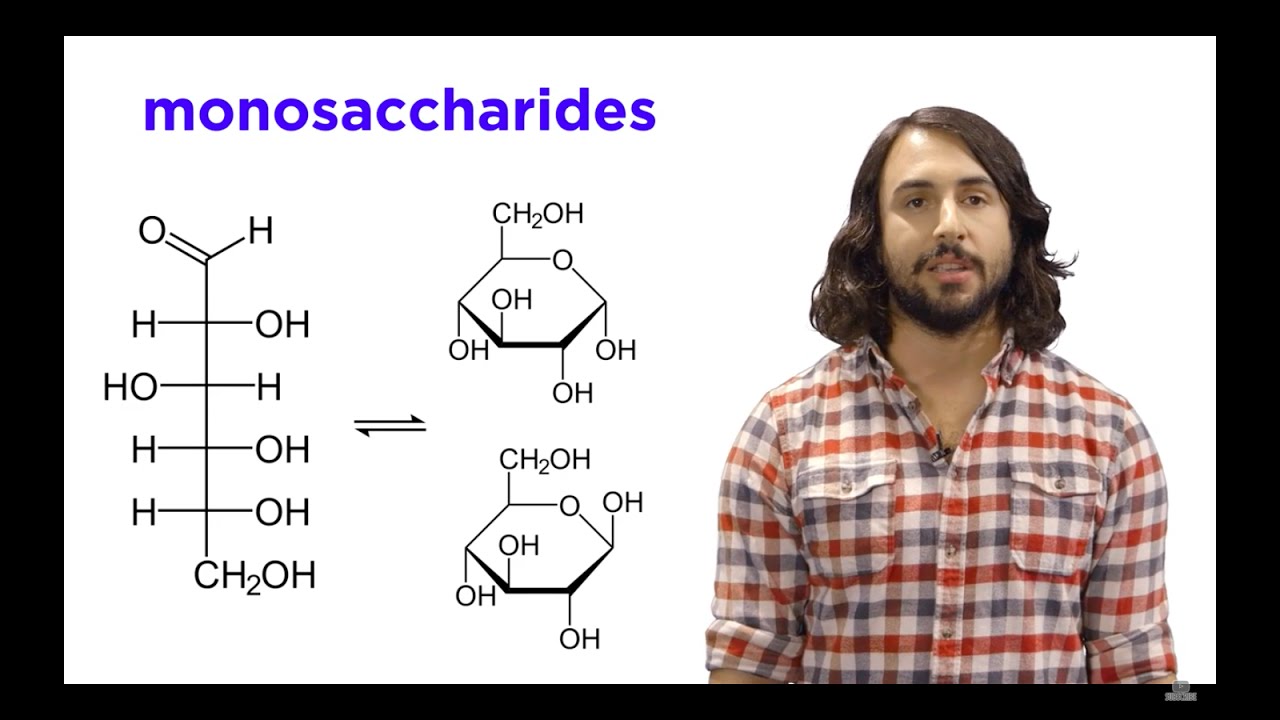
Fischer to Haworth shortcut for Glucose and Fructose
Summary
TLDRThis video tutorial provides an easy-to-understand guide for converting D-Glucose and D-Fructose between Fischer projections, Haworth projections, and chair conformations. The video breaks down the conversion process with step-by-step instructions, including key concepts like chiral carbons, nucleophilic attack, and ring formation. It also includes tips on how to visualize the structures without confusion, using mnemonic devices and shortcuts for both glucose and fructose. The tutorial emphasizes the importance of knowing the structural details for MCAT preparation and highlights the differences between alpha and beta anomers.
Takeaways
- 😀 Fischer Projections represent 3D chiral molecules in 2D, allowing easy depiction of sugars like D-Glucose and D-Fructose.
- 😀 D-Glucose is an Aldohexose, meaning it has an aldehyde group and six carbon atoms, whereas D-Fructose is a Ketohexose, with a ketone group and six carbons.
- 😀 For D-Glucose, the chiral carbons follow a 'Right, Left, Right' pattern in its Fischer projection.
- 😀 In D-Glucose, the hydroxyl group (OH) on the lowest chiral carbon (C5) is on the right, which differentiates it from L-Glucose where it is on the left.
- 😀 The mechanism of converting D-Glucose to its Haworth projection involves a nucleophilic attack from the oxygen on C5 to the carbonyl carbon (C1), forming a 6-membered pyranose ring.
- 😀 In Haworth projections, the anomeric carbon (C1) determines the alpha or beta configuration, with the OH group pointing down for alpha and up for beta.
- 😀 For D-Fructose, the nucleophilic attack happens at C2 (the ketone), resulting in a smaller 5-membered furanose ring.
- 😀 In the Haworth projection of D-Fructose, the OH group on C2 determines the alpha or beta anomeric configuration, similar to D-Glucose.
- 😀 When converting Fischer projections to Haworth, it's essential to remember that for D-sugars, the CH2OH group on C6 will be up in the Haworth ring.
- 😀 The process of converting Fischer to Haworth projections can be simplified by following a consistent rule: drop carbons 2, 3, and 4 down and place carbon 5 (with the CH2OH group) up.
- 😀 D-Fructose can form either a furanose (5-membered) or pyranose (6-membered) ring, but the furanose ring is more commonly observed when C5 attacks the ketone at C2.
Q & A
What is the difference between D-Glucose and D-Fructose in terms of their structure?
-D-Glucose is an aldohexose, meaning it has an aldehyde group at position 1 and a six-carbon backbone, while D-Fructose is a ketohexose, with a ketone group at position 2 and also a six-carbon backbone.
What is the main feature of Fischer projections in representing sugars?
-Fischer projections represent the 3D structure of chiral molecules in 2D. The horizontal lines represent bonds that point out of the plane, while vertical lines represent bonds that are going behind the plane.
How does one determine the configuration (D or L) of glucose in a Fischer projection?
-To determine if glucose is D or L, observe the orientation of the hydroxyl group (OH) on the lowest chiral carbon. For D-Glucose, the OH is on the right side of this carbon, while for L-Glucose, it is on the left.
What is the mnemonic for remembering the orientation of hydroxyl groups in Fischer projections?
-The mnemonic is 'Right, Left, Right' for D-Glucose, where the OH groups on the chiral carbons alternate between right and left positions.
What is the significance of the carbonyl group in the interconversion between linear and cyclic forms of glucose?
-The carbonyl group (aldehyde in glucose) reacts with a hydroxyl group on carbon 5 to form a cyclic structure. This reaction, where carbon 1 becomes the anomeric carbon, leads to the formation of a pyranose ring.
How is the Haworth projection of glucose simplified?
-In the Haworth projection, the cyclic structure is shown flat, with the ring’s atoms represented by a simple, simplified skeletal diagram. The direction of the substituents is based on the original Fischer projection, with the trick 'drop it down, left up' to determine the positions of the groups.
Why is carbon 5 in D-Glucose tricky when drawing its Haworth projection?
-Carbon 5 is tricky because when the OH group on carbon 5 attacks carbon 1 to form the ring, it causes a rotation in the molecule. This results in a different orientation for the CH2OH group, which must be accounted for when drawing the projection.
What is the rule for determining the anomeric configuration (alpha or beta) in the Haworth projection?
-In the Haworth projection, if the OH group on the anomeric carbon (carbon 1) points down, the configuration is alpha; if it points up, the configuration is beta.
What is the difference between the pyranose and furanose forms of sugars?
-The pyranose form is a six-membered ring, like in D-Glucose, while the furanose form is a five-membered ring, like in D-Fructose when carbon 5 attacks the carbonyl group at position 2.
How can you remember the orientation of the CH2OH group in D-Glucose's Haworth projection?
-For D-Glucose, the CH2OH group on carbon 6 always goes up in the Haworth projection, while for L-Glucose, the CH2OH group on carbon 6 would go down.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen
5.0 / 5 (0 votes)