What is entropy? - Jeff Phillips
Summary
TLDRThis video explains the concept of entropy, a fundamental idea in chemistry and physics that helps clarify why physical processes occur in one direction, such as ice melting and cream mixing in coffee. Rather than merely being a measure of disorder, entropy is linked to the probability of energy configurations. It highlights that energy tends to spread out into more probable states, demonstrating why spontaneous processes lead to higher entropy. The discussion emphasizes that while lower entropy states are possible, they are statistically unlikely, making high entropy a natural tendency in energy distribution.
Takeaways
- 😀 Entropy is a key concept in chemistry and physics that explains the direction of physical processes.
- 🌡️ Entropy is often described as a measurement of disorder, but this interpretation can be misleading.
- 🔄 The concept of entropy can be better understood through the idea of microstates and energy distribution.
- ⚛️ Each energy configuration of a system can exist in many different arrangements, known as microstates.
- 📈 Higher entropy corresponds to energy configurations that are more spread out, indicating a greater number of microstates.
- 🔥 Hot objects tend to cool down while cold objects warm up due to the statistical likelihood of energy spreading out.
- 🔍 In small systems, spontaneous increases in energy (like a hot object getting hotter) are statistically possible but highly unlikely.
- 📏 Real-world objects have an enormous number of particles, making high-entropy states far more likely than low-entropy states.
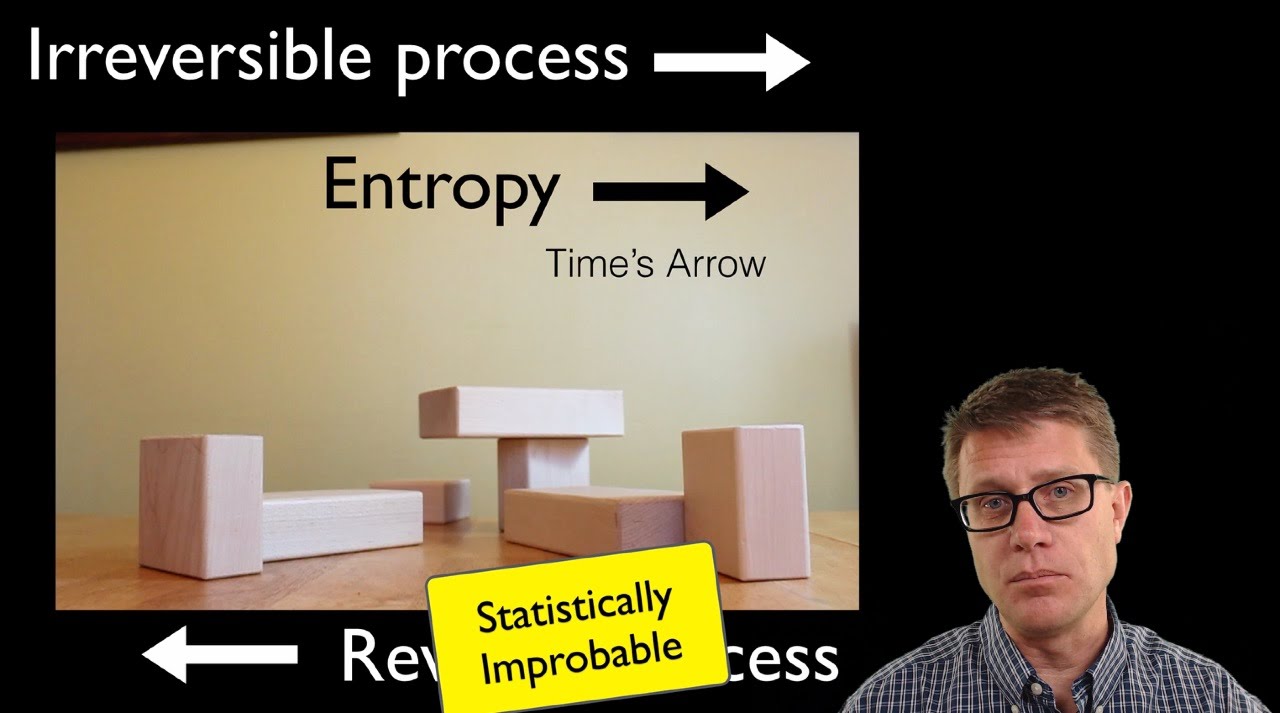
- ⏳ The tendency of energy to spread out is why entropy is referred to as time's arrow.
- 🔗 No mysterious force drives systems toward higher entropy; it is simply a matter of statistical probability.
Q & A
What is entropy and why is it significant in chemistry and physics?
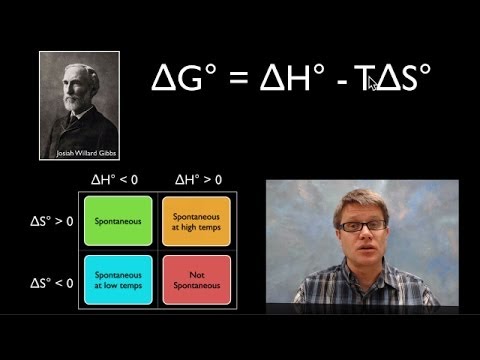
-Entropy is a measure of the distribution of energy in a system. It explains the direction of physical processes, such as why hot objects cool down and why ice melts.
Why is the common description of entropy as 'disorder' misleading?
-Describing entropy as disorder is misleading because it does not accurately represent the statistical nature of energy configurations; a more effective description involves probability and energy distribution.
How is entropy related to microstates?
-Entropy is a measure of the number of possible microstates associated with a particular energy configuration. More microstates correspond to higher entropy.
What example does the script use to explain microstates?
-The script uses two solids with atomic bonds containing energy quanta to illustrate how different configurations can represent the same total energy, leading to various microstates.
What is the relationship between energy distribution and entropy?
-Energy configurations with more spread out energy have higher entropy because there are more ways (microstates) to arrange the energy in such a dispersed manner.
What happens to energy in a dynamic system according to the script?
-In a dynamic system, energy continuously moves between neighboring bonds, allowing the energy configuration to change and contributing to entropy.
Why is it statistically unlikely for a hot object to become hotter when placed next to a cold one?
-The probability of a hot object gaining energy is extremely small due to the vast number of microstates that favor energy dispersion, making spontaneous heating virtually impossible.
What does the script imply about spontaneous processes?
-Spontaneous processes tend to favor states of higher entropy, meaning systems will naturally evolve toward configurations where energy is more dispersed.
How does the concept of 'time's arrow' relate to entropy?
-Entropy is often referred to as 'time's arrow' because it indicates the direction of spontaneous processes; as time progresses, systems tend to evolve toward higher entropy states.
Why is there no mysterious force causing systems to move toward higher entropy?
-There is no mysterious force at play; systems naturally evolve towards higher entropy because those states are statistically more likely due to the greater number of accessible microstates.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführen5.0 / 5 (0 votes)