What is the shape of a molecule? - George Zaidan and Charles Morton
Summary
TLDRThis script explores the concept of molecular shape, emphasizing that molecules are not solid like statues but consist mostly of empty space with dense atomic nuclei and electron clouds. It explains how molecules arrange to maximize charge attraction and minimize repulsion, using methane as an example to illustrate the tetrahedron shape. The script also mentions various molecular shapes like pyramids, lines, and letters, highlighting the complexity as atoms increase and the potential for multiple stable configurations.
Takeaways
- 🌌 A molecule is mostly empty space, with mass concentrated in dense atomic nuclei and electrons forming clouds of negative charge.
- 🔬 Molecules do not have a fixed shape like a statue, but they have optimal arrangements to balance attraction and repulsion between charges.
- 🌀 The outermost electrons determine the molecular shape, and electron clouds between atoms resemble sausages.
- 🔋 Alessandro Volta discovered methane (CH4) in 1776, which is composed of one carbon and four hydrogen atoms.
- 🔗 Carbon can bond with up to four other atoms, and each hydrogen can bond with one other atom, suggesting a central carbon atom in methane.
- 📐 Each bond in a molecule represents the sharing of two electrons, which can be represented as lines in a flat diagram.
- 🔵 The optimal 3D arrangement of methane's atoms forms a tetrahedron to maximize the distance between electron clouds.
- 🔄 Different molecules can have various shapes, such as pyramids, straight lines, bends, or the letter T.
- 🧪 Experimental evidence is necessary to confirm the predicted shapes of molecules.
- 🔄 Molecules can have multiple stable arrangements, leading to interesting chemistry when they switch between configurations.
Q & A
What is the primary reason molecules don't have a fixed shape like a statue?
-Molecules don't have a fixed shape because they consist mostly of empty space with mass concentrated in dense nuclei and electrons existing as diffuse clouds of negative charge rather than discrete particles.
Why is the arrangement of atoms in a molecule important?
-The arrangement of atoms in a molecule is important because it determines the molecule's shape, which in turn influences its chemical properties and reactivity.
What role do electrons play in shaping a molecule?
-Electrons, particularly the valence electrons, play a crucial role in shaping molecules because they are involved in forming bonds between atoms and their distribution affects the overall molecular geometry.
How does the concept of electron clouds relate to molecular shape?
-Electron clouds, which are regions of negative electric charge, influence molecular shape by repelling each other, leading to an arrangement that maximizes the distance between bonds.
What is the significance of Alessandro Volta's discovery of methane in relation to molecular structure?
-Alessandro Volta's discovery of methane highlights the importance of understanding molecular structure beyond just the types of atoms present, emphasizing the need to know how these atoms are bonded and arranged in space.
What is the chemical formula of methane, and what does it imply about its molecular structure?
-The chemical formula of methane is CH4, indicating that it consists of one carbon atom bonded to four hydrogen atoms, but it does not specify the three-dimensional arrangement of these atoms.
Why is carbon considered the central atom in methane?
-Carbon is considered the central atom in methane because of its ability to form four bonds, as indicated by its electron configuration, which allows it to bond with all four hydrogen atoms.
What is the optimal geometric shape for methane based on the repulsion of electron clouds?
-The optimal geometric shape for methane is a tetrahedron, which allows the four bonds to be as far apart as possible, minimizing repulsion between the electron clouds.
Can you provide examples of different molecular shapes for different molecules?
-Yes, ammonia (NH3) forms a pyramidal shape, carbon dioxide (CO2) is linear, water (H2O) is bent, and chlorine trifluoride (ClF3) has a T-shape.
How do experiments confirm the predicted shapes of molecules?
-Experiments such as X-ray crystallography, NMR spectroscopy, and electron diffraction can be used to determine the actual three-dimensional structure of molecules, confirming or refuting theoretical predictions.
What is the significance of molecules having multiple stable arrangements of atoms?
-Molecules with multiple stable arrangements can undergo isomerism, which is crucial for understanding their chemical behavior and can lead to interesting chemical reactions and properties.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen

Modelo Atômico de Rutherfod | Atomística | Química Geral

Geometria Molecular | Teoria VSEPR | Teoria de Repulsão dos Pares de Elétrons da Camada de Valência

Química Simples #47 - [Ligações] - Geometria Molecular (parte 1/3)
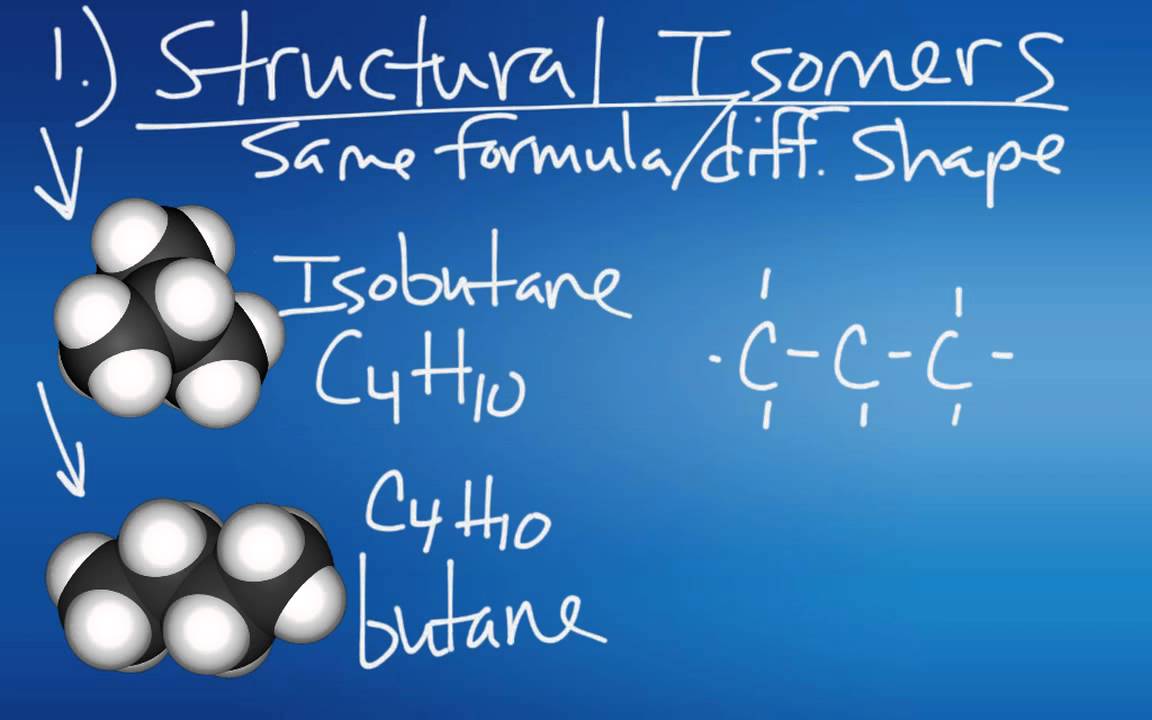
Isomers

แบบจำลองอะตอมของรัทเทอร์ฟอร์ด

Rutherford's Gold Foil Experiment - Quick and Simple!
5.0 / 5 (0 votes)
