Water Potential
Summary
TLDRThis video explains water potential, the potential energy of water relative to pure water, and how it determines water movement in biological systems. The presenter discusses osmosis, solute potential, and pressure potential, using examples like salt on a slug and water flowing in trees. Key equations are introduced, such as solute potential (ψs = -iCRT), with steps to calculate it in problems. The video emphasizes how water moves from high to low potential, influenced by solute concentration, pressure, and temperature, and concludes with an example calculation.
Takeaways
- 💧 Water potential refers to the potential energy of water per unit area compared to pure water and determines the direction water will flow.
- 🔄 Water potential is measured using the Greek letter psi (Ψ), which is reminiscent of Poseidon's trident.
- 🧂 Water potential consists of two main components: solute potential (Ψs) and pressure potential (Ψp).
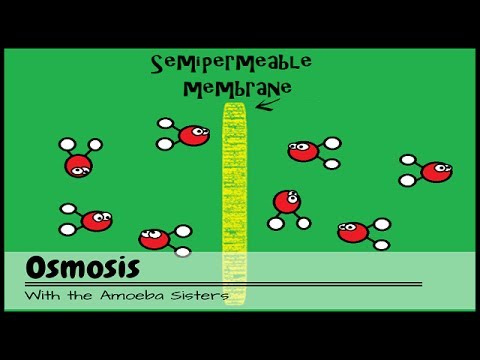
- 🧬 During osmosis, water moves from areas of high water potential to areas of low water potential.
- 🐌 Pouring salt on a slug creates a lower water potential outside the slug, drawing water out and causing the slug to shrivel.
- 🌿 Water moves up a tree along a water potential gradient, from the roots (higher water potential) to the leaves (lower water potential).
- 🔢 The overall water potential is the sum of solute potential (affected by the number of solutes) and pressure potential (caused by physical pressure).
- 🧪 The formula for solute potential is Ψs = -iCRT, where i is the ionization constant, C is the concentration, R is the pressure constant (0.0831), and T is temperature in Kelvin.
- 🍬 Substances like sucrose have an ionization constant of 1 since they do not break into ions when dissolved in water.
- 📏 When calculating water potential in an open beaker, the pressure potential is zero, so the water potential equals the solute potential.
Q & A
What is water potential and how is it defined?
-Water potential is the potential energy of water per unit area, compared to pure water. It helps predict the direction of water movement, driven by osmosis, gravity, pressure, or surface tension.
How does osmosis affect water potential in the context of a slug?
-Osmosis causes water to move from an area of higher water potential (inside the slug) to an area of lower water potential (where salt is applied on the slug's surface), leading to water being drawn out of the slug, which can cause it to shrivel.
How does the dissociation of sodium chloride in water affect water potential?
-When sodium chloride dissolves in water, it separates into sodium and chloride ions. These ions are surrounded by water molecules, which decreases the water potential by creating more areas for water to move into.
How does water potential drive water movement in plants?
-Water moves from areas of higher water potential, like the soil (0 bars), to areas of lower water potential, such as the roots, stems, and leaves. The gradient is driven by osmosis and evaporation, allowing water to move up through the plant.
What factors contribute to overall water potential?
-Water potential is determined by two main factors: solute potential (which decreases with the addition of solutes) and pressure potential (which increases with physical pressure exerted by the cell wall in plants).
How do solutes affect solute potential and overall water potential?
-Adding solutes, such as sodium chloride, decreases solute potential because it opens spaces for water to move into, resulting in lower overall water potential.
What role does pressure potential play in water movement in plant cells?
-Pressure potential is the physical force exerted by the cell wall when water enters the cell. It resists further water influx by exerting pressure, which can prevent cells from bursting.
What is the equation for solute potential, and what do the variables represent?
-The equation for solute potential is -iCRT, where 'i' is the ionization constant (number of particles a solute dissociates into), 'C' is the molar concentration, 'R' is the pressure constant (0.0831), and 'T' is the temperature in Kelvin.
Why is it important to convert temperature to Kelvin in the solute potential equation?
-Temperature must be converted to Kelvin to ensure correct calculations in the solute potential equation. This is done by adding 273 to the Celsius temperature.
How do you calculate water potential in an open beaker scenario?
-In an open beaker, the pressure potential is 0 bars, so the overall water potential is equal to the solute potential. If the solute potential is calculated to be -5 bars, the water potential will also be -5 bars.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführen5.0 / 5 (0 votes)