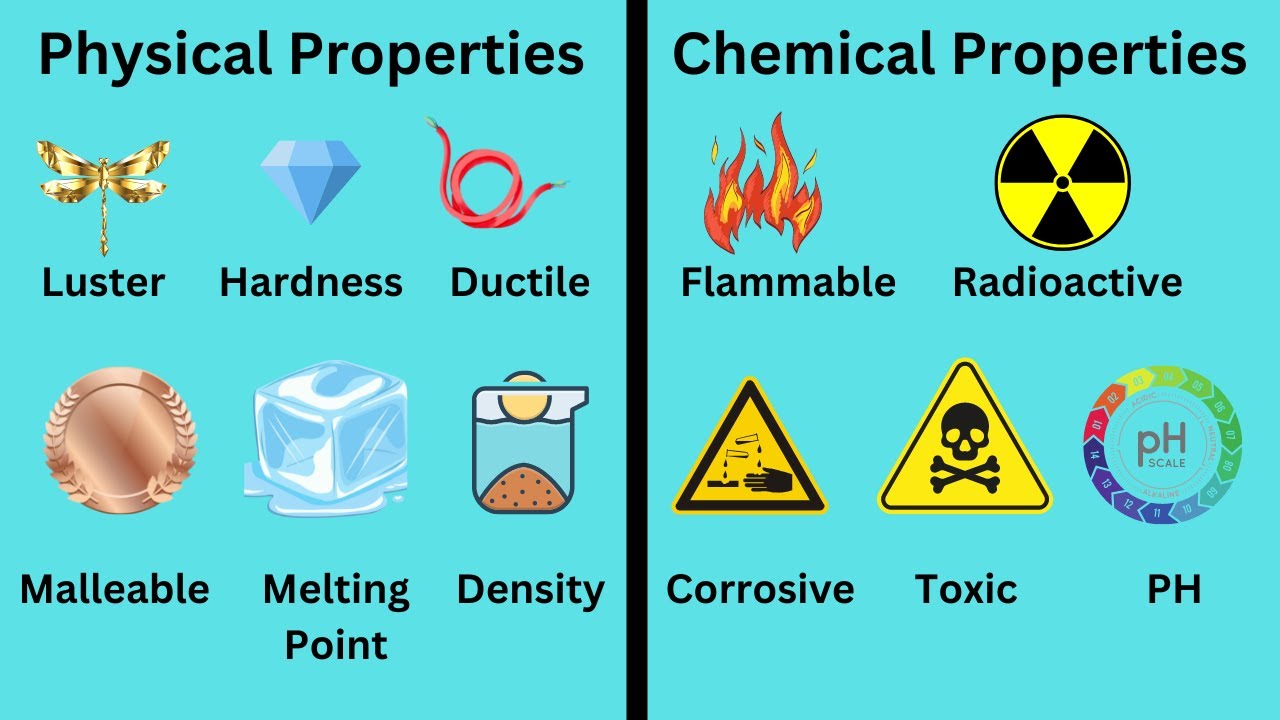
PROPIEDADES DE LA MATERIA. CONCEPTO Y CLASES.
Summary
TLDRThis video explains the fundamental properties of matter in chemistry, covering topics like mass, volume, weight, density, porosity, and specific properties such as hardness, malleability, and ductility. It highlights key concepts such as the distinction between mass and weight, the effects of gravity, and the behavior of materials under different conditions. The video also delves into more specialized concepts like the solubility of substances and their ability to undergo physical changes like melting or boiling. Through simple examples and illustrations, the video provides an easy-to-understand introduction to these essential chemistry concepts.
Takeaways
- 😀 Matter is anything that has mass and occupies space, including objects, air, planets, and even humans.
- 😀 Mass refers to the amount of matter in an object and is measured in kilograms using balances.
- 😀 Volume is the amount of space an object occupies, measured in liters for liquids and cubic centimeters for solids.
- 😀 Weight is the force with which gravity pulls on an object and depends on the gravitational field of the planet.
- 😀 Porosity is the amount of empty space within a material, affecting its ability to absorb or pass substances like liquids or gases.
- 😀 Inertia is the resistance of a chemical species to react, representing chemical stability.
- 😀 Impenetrability means that two objects cannot occupy the same space simultaneously, as shown by a stone displacing water.
- 😀 Elasticity is the ability of a material to return to its original shape after being stretched or deformed.
- 😀 Specific properties, like hardness and malleability, help differentiate one material from another and depend on the material's composition.
- 😀 Density is the mass per unit volume of a substance, and it helps explain why honey is denser than water.
- 😀 The boiling point is the temperature at which a substance changes from liquid to gas, with water boiling at 100°C and mercury at 356.6°C.
Q & A
What is matter?
-Matter is anything that has mass and occupies space. This includes physical objects like a computer, book, chair, as well as intangible things like air.
How do we measure mass?
-Mass is measured in kilograms using balance scales. There are mechanical balances like the granat balance and digital balances that are commonly used in laboratories.
What is the difference between mass and weight?
-Mass is the amount of matter in an object, and it remains constant regardless of location. Weight, on the other hand, is the force of gravity on an object, which can change depending on the gravitational field of the planet.
What is volume, and how is it calculated?
-Volume is the amount of space an object occupies. For solids, volume is calculated as length × width × height. For liquids, volume is measured in liters or milliliters.
What is porosity, and why is it important?
-Porosity refers to the percentage of empty spaces within a material that allow liquids or gases to pass through. It is important in fields like geology and material science because it determines how well materials like rocks can absorb or pass water.
What is inertia in chemistry?
-Inertia refers to a substance's resistance to undergoing chemical reactions. It is often associated with molecules that have strong bonds and are chemically stable, like noble gases or nitrogen.
What does impenetrability mean in terms of matter?
-Impenetrability is the property that prevents two bodies from occupying the same space at the same time. For example, when you place a stone in a glass of water, the stone displaces the water, showing that both cannot exist in the same space.
What is elasticity in materials?
-Elasticity is the ability of a material to return to its original shape after being stretched or compressed. A rubber band is a common example of an elastic material.
What is the difference between malleability and ductility?
-Malleability is the ability of a material to be shaped into thin sheets without breaking, while ductility is the ability of a material to be drawn into thin wires without breaking. Both are types of plastic deformation.
How do the melting and boiling points of substances differ?
-The melting point is the temperature at which a solid turns into a liquid, while the boiling point is the temperature at which a liquid turns into a gas. Each substance has its unique melting and boiling points, depending on its molecular structure.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen
5.0 / 5 (0 votes)