Why Uranium Enrichment is a Big Deal
Summary
TLDRThis video explains the basics of uranium enrichment, diving into the science of isotopes, nuclear reactions, and centrifuges. It starts with an introduction to uranium, its isotopes, and their role in nuclear reactions. The process of enriching uranium 235 to make it suitable for different purposes—ranging from nuclear power to nuclear weapons—is broken down. The explanation covers the mechanics of centrifuges, which separate uranium isotopes by exploiting differences in their masses. The video highlights how the simple concept of a centrifuge, used in various fields, has significant geopolitical implications, providing a cosmic perspective on this powerful technology.
Takeaways
- 😀 Uranium is a heavy element with 92 protons, making it the heaviest naturally occurring element.
- 😀 While uranium is the heaviest natural element, humans have created elements heavier than uranium using particle accelerators, with the heaviest being element 118.
- 😀 Uranium occurs naturally in ores as a mixture of three isotopes: U234, U235, and U238, all of which have 92 protons but different numbers of neutrons.
- 😀 Isotopes are variations of elements where the number of neutrons differs, but the number of protons remains the same.
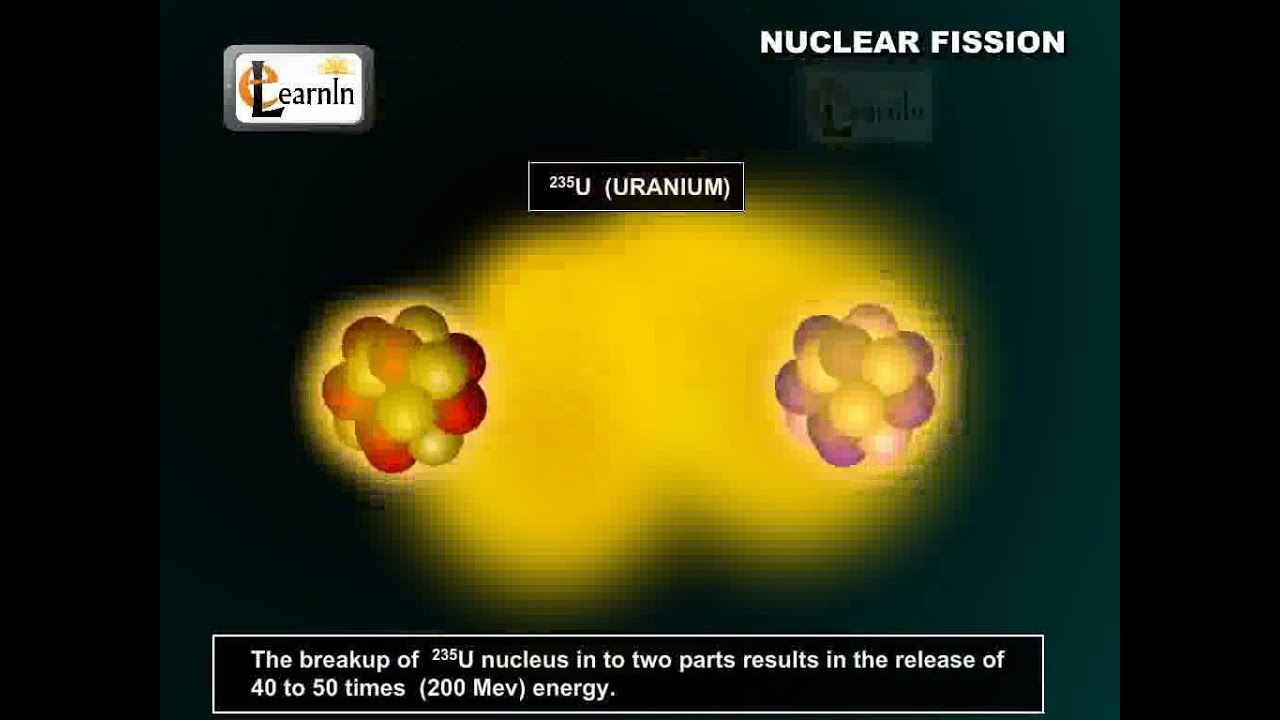
- 😀 Uranium atoms, especially U235, are unstable and can undergo fission, splitting into two lighter atoms and releasing energy.
- 😀 Uranium-235 can undergo a chain reaction when split, releasing more neutrons that cause further fission, amplifying the process exponentially.
- 😀 U234 and U238 do not exhibit the same chain reaction properties as U235, making U235 the most useful isotope for energy production and weaponization.
- 😀 To produce enriched uranium (mainly U235), centrifuges are used to separate U235 from U234 and U238 by spinning the material at very high speeds.
- 😀 Centrifuges are capable of creating extreme centrifugal forces (up to a million G's) to effectively isolate U235 for use in energy production or weapons.
- 😀 The level of uranium enrichment needed depends on its intended use: 5% enrichment for nuclear power plants, around 20% for nuclear propulsion, and over 90% for nuclear weapons.
- 😀 Even a simple centrifuge, used in medical applications, can play a significant role in influencing geopolitical power when used for uranium enrichment, highlighting the global implications of nuclear technology.
Q & A
What is uranium, and how is it classified on the periodic table?
-Uranium is a chemical element with 92 protons. It is traditionally considered the heaviest naturally occurring element on the periodic table.
What is the significance of the number of protons in an element?
-The number of protons in an element defines what element it is. For example, uranium always has 92 protons, distinguishing it from other elements.
How have humans gone beyond the natural elements found in the universe?
-Humans have used particle accelerators to bombard the nuclei of elements, creating new elements heavier than uranium, with the current heaviest being element 118.
What are isotopes, and how do they relate to uranium?
-Isotopes are variations of an element that have the same number of protons but a different number of neutrons. Uranium has several isotopes, including Uranium-234, Uranium-235, and Uranium-238.
Why are uranium isotopes unstable?
-Uranium isotopes, particularly Uranium-235, have a large number of neutrons in their nuclei, making them unstable and prone to spontaneous fission, where the nucleus breaks apart into lighter elements.
How does nuclear fission work in Uranium-235?
-When Uranium-235 is hit by a neutron, its nucleus splits into two lighter atoms, releasing energy and additional neutrons, which can trigger a chain reaction.
What is a chain reaction, and why is it important in nuclear reactions?
-A chain reaction occurs when the splitting of one atom releases neutrons that cause further splitting in other atoms. This process exponentially increases the number of reactions and releases a large amount of energy, which is crucial for both nuclear power generation and nuclear weapons.
Why is Uranium-235 used for nuclear bombs, but not the other uranium isotopes?
-Uranium-235 is the only isotope that, when split, produces extra neutrons, making it capable of sustaining a chain reaction. Uranium-234 and Uranium-238 do not have this property, making them unsuitable for weapons.
What is uranium enrichment, and why is it necessary?
-Uranium enrichment is the process of increasing the concentration of Uranium-235 in uranium ore. Since only about 1% of mined uranium is Uranium-235, enrichment is needed to isolate enough of it for practical uses like nuclear power or weapons.
How do centrifuges work in the process of uranium enrichment?
-Centrifuges separate uranium isotopes by spinning uranium gas at high speeds, using centrifugal force to separate the denser Uranium-238 from the lighter Uranium-235.
Why is uranium gas used instead of solid uranium for centrifuging?
-Uranium needs to be in a gas form for centrifuging because centrifuges cannot separate solid matter. Gaseous uranium allows the centrifugal forces to effectively distinguish between the isotopes based on their slight mass difference.
What are the different levels of uranium enrichment, and what are they used for?
-Uranium enrichment levels vary: 5% is used for nuclear power plants, 20% is used for nuclear propulsion (e.g., submarines), and 90% or more is required for nuclear weapons.
How powerful are the centrifuges used for uranium enrichment?
-The centrifuges used for enriching uranium can spin at speeds that generate up to a million G’s, which is necessary to separate Uranium-235 from Uranium-238 effectively.
How does the concept of a centrifuge relate to everyday experiences?
-A simple analogy is that Earth acts as a giant centrifuge due to gravity, causing denser materials (like salad dressing) to settle at the bottom while lighter substances rise to the top.
What broader impact does centrifuge technology have beyond nuclear applications?
-Centrifuge technology has applications beyond nuclear science, such as in medical labs where it is used to separate blood components. Additionally, it plays a significant role in global geopolitical power, as nations with the capability to enrich uranium gain strategic leverage.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة

ЯДЕРНАЯ ПРОГРАММА ИРАНА: ВСЯ ПРАВДА. Готов ли Тегеран к ЯДЕРНОЙ БОМБЕ?

How ASP Isotopes is Revolutionizing the Global Isotope Supply Chain

Difference between Nuclear Fission and Nuclear Fusion

Nuclear Fission and Radioactivity - Part 1 of 3
Physics - Nuclear Fission reaction explained - Physics

Langkah Membuat Senjata Nuklir
5.0 / 5 (0 votes)
