BALANCEAMENTO de Equações por TENTATIVAS (Método Rápido MACHO) + Exercícios | Aula 01 (Química IV)
Summary
TLDRIn this Chemistry 4 class, Professor Marcelo introduces students to the basics of chemistry, focusing on understanding the periodic table and balancing chemical equations using the trial method. The lesson highlights the importance of the periodic table and guides beginners through the process of balancing equations, emphasizing a systematic approach. With practical examples and clear explanations, the class covers various elements like metals, chlorine, carbon, hydrogen, and oxygen. Marcelo offers step-by-step instructions, tips, and alternative solutions to ensure comprehension, while encouraging students to practice with additional exercises linked in the video description.
Takeaways
- 😀 Understanding the periodic table is essential for balancing chemical equations.
- 😀 The method of balancing chemical equations involves using a trial and error approach, starting with metals and moving through non-metals like carbon, hydrogen, and oxygen.
- 😀 A key rule in balancing equations is the 'man's rule,' which suggests balancing metals first, followed by carbon, hydrogen, and oxygen.
- 😀 In a chemical equation, the reactants are on the left side, and the products are on the right side of the equation.
- 😀 The principle of conservation of mass means that no atoms are created or destroyed during a chemical reaction, which requires balancing the equation correctly.
- 😀 The index (subscript) in a chemical formula indicates the number of atoms of that element in a molecule, which helps in balancing the equation.
- 😀 Coefficients are used to balance equations and should be placed in front of the compounds, not below them.
- 😀 The balancing process includes calculating the appropriate coefficients by dividing the larger number by the smaller one to achieve the correct balance.
- 😀 Special cases, like parentheses in chemical formulas, require multiplying the subscripts inside the parentheses by the coefficient outside to ensure proper balancing.
- 😀 Fractional coefficients can be used in certain cases but are not preferred in most chemistry classes because they can complicate the process.
- 😀 Practice is essential for becoming proficient at balancing equations, and students are encouraged to work through exercises provided in the video description.
Q & A
What is the primary focus of this chemistry class?
-The primary focus is on balancing chemical equations using the method of trials, specifically addressing students who are new to chemistry and may be unfamiliar with the periodic table.
How does Professor Marcelo explain the importance of the periodic table in this class?
-Professor Marcelo emphasizes that the periodic table is crucial for understanding the positioning of metals and other elements, which helps in balancing chemical equations. He also provides a link to the UERJ periodic table for students to download and refer to.
What is the method of balancing equations discussed in the video?
-The method of trials is introduced, where students follow a specific order of balancing elements, starting with metals and progressing through carbon, hydrogen, and oxygen. This method involves trial and error to find the correct coefficients.
What is the 'man's rule' for balancing chemical equations?
-The 'man's rule' suggests that when balancing equations, you first balance metals, then carbon, hydrogen, and finally oxygen. This systematic approach helps in the trial and error method.
What is the purpose of the coefficients in a chemical equation?
-Coefficients are used to balance the equation by ensuring that the number of atoms of each element is the same on both sides of the equation. They are determined by dividing the larger number of atoms by the smaller number.
How does the concept of 'reactants' and 'products' apply to a chemical equation?
-In a chemical equation, reactants are the substances on the left side, and products are the substances on the right side. The goal is to balance them by ensuring the number of atoms of each element is equal on both sides.
What does Professor Marcelo mean when he says 'in nature, nothing is created, nothing is lost, everything is transformed'?
-This phrase refers to the law of conservation of mass, which states that in any chemical reaction, the total mass of the reactants must equal the total mass of the products, implying no atoms are lost or created.
How are fractional coefficients handled in balancing equations?
-Fractional coefficients, such as 0.5, are sometimes used to balance equations when whole numbers aren't sufficient. However, it's noted that many entrance exams prefer not to use fractions, and alternatives like multiplying all coefficients to avoid fractions may be considered.
What is the significance of the exercises provided in the video description?
-The exercises provide additional practice for students, allowing them to reinforce what they've learned in the video. They are designed to be used for practice and to help students gain speed in balancing equations.
What advice does Professor Marcelo give to students who are just starting to learn how to balance equations?
-Professor Marcelo advises students to start slowly and carefully, following each step to understand the process. He encourages them to practice using the provided exercises and gain speed over time without skipping steps.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
How to Write Chemical Equations

Balanceamento de Equações Químicas - Brasil Escola

All of AQA CHEMISTRY Paper 1 in 30 minutes - GCSE Science Revision

1. Introdução ao Estudo da Química [Química Geral]

Video Pembelajaran Model Problem Based Learning Materi Persamaan Reaksi Kimia
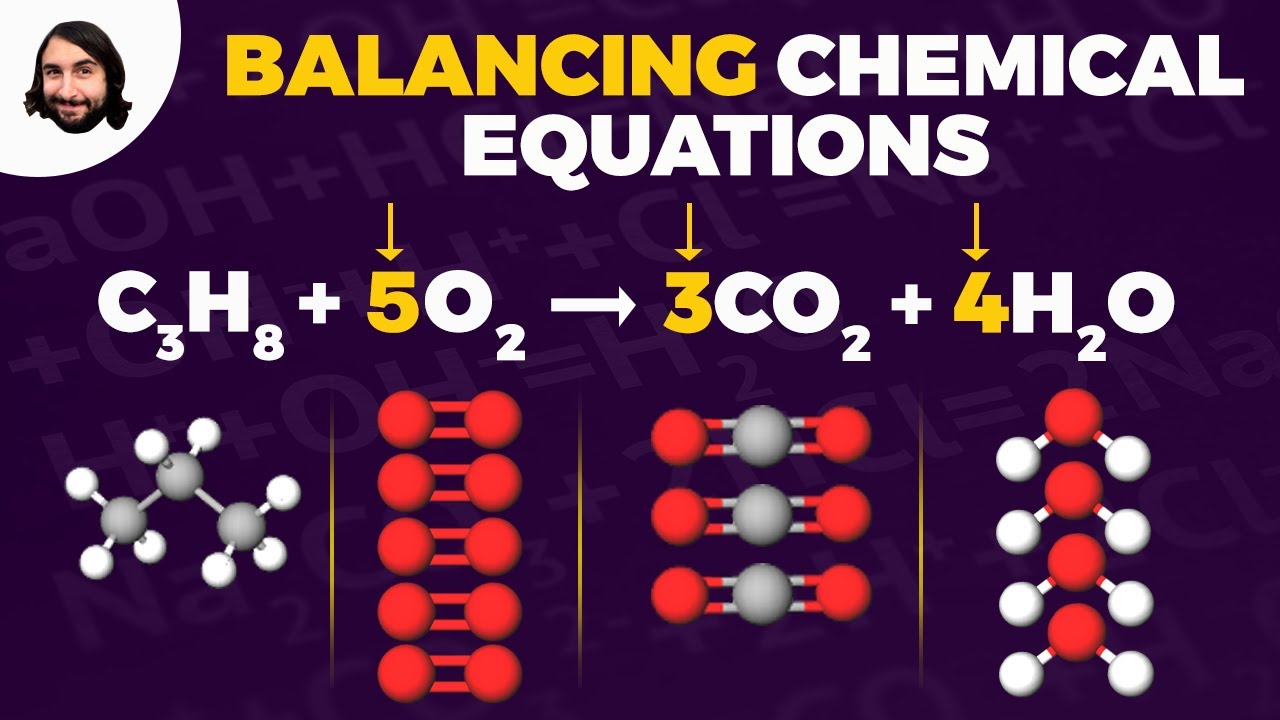
Balancing Chemical Equations
5.0 / 5 (0 votes)
