Como funciona um espectrofotômetro?
Summary
TLDRThe spectrophotometer is a crucial instrument in laboratory analysis, used to determine the composition and concentration of materials based on the light they absorb or transmit. The device uses a light source, a prism to decompose the light spectrum, and a monochromator to select specific wavelengths. The light passes through a sample in a cuvette, and the absorbance or transmittance is measured by the detector. This data helps identify the concentration of solutes in a solution. The spectrophotometer is valuable in environmental analysis, such as determining the presence of organic and inorganic compounds like pesticides and heavy metals, or analyzing the iron concentration in water samples.
Takeaways
- 😀 The spectrophotometer is a laboratory device used to determine the composition and concentration of materials by analyzing how much light is absorbed or transmitted by a sample.
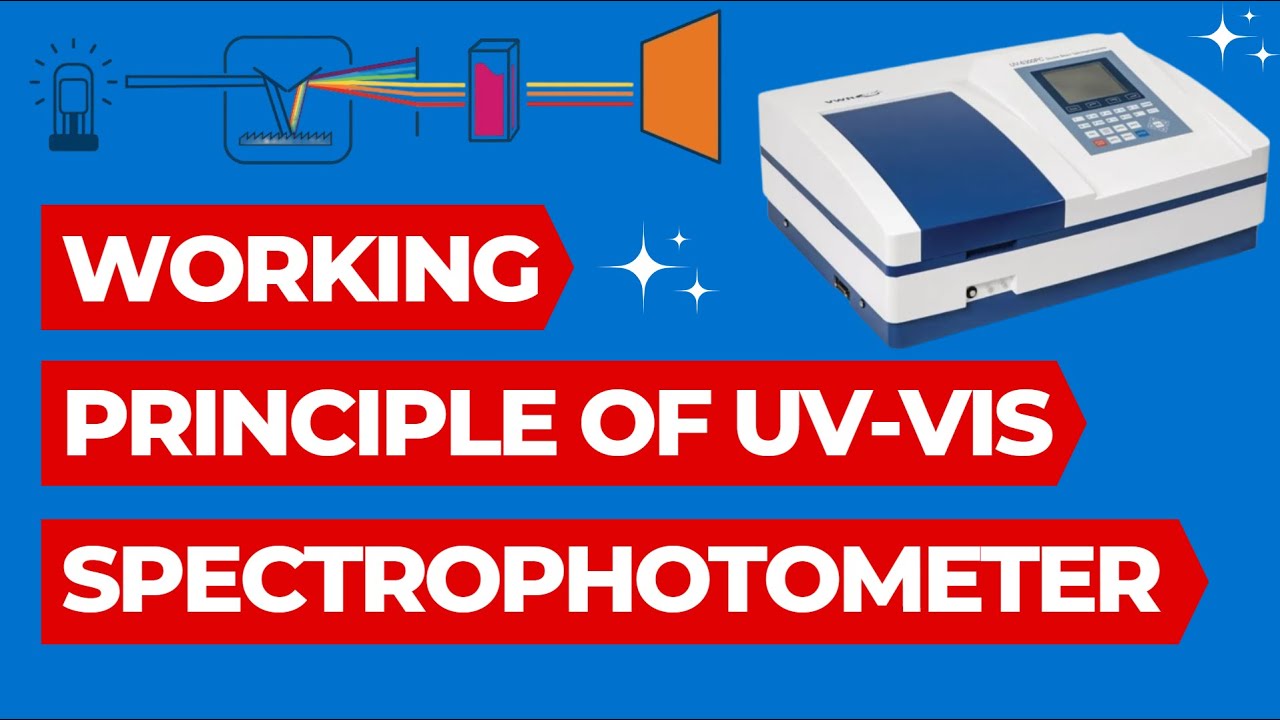
- 🌈 The device works by passing white light through a prism that separates it into its component colors: red, orange, yellow, green, blue, violet, including infrared and ultraviolet wavelengths.
- 🔴 The closer the light is to red, the lower the frequency and the longer the wavelength, and the device allows for specific wavelength selections using a monocromator.
- 🔬 The sample is placed in a cuvette, where the light either gets absorbed by the molecules in the solution or transmitted through to a detector.
- 💡 The absorbance is the amount of light absorbed by the solution, and the transmitted light is measured by the detector, which calculates the concentration of the substance based on the light that passes through.
- 📊 The software of the spectrophotometer analyzes the transmitted light to determine how much light was absorbed and compares this with other samples to determine the solute concentration.
- 🌍 The spectrophotometer is widely used in environmental water analyses to detect organic and inorganic compounds, such as pesticides and heavy metals.
- 💧 An example use case involves determining the amount of dissolved iron in water, where a reagent is added to the sample to create a color change.
- 🧪 To determine iron concentration, solutions with known concentrations of reagents are prepared, and these are compared with the water sample to establish concentration levels.
- ⚙️ After adjusting the spectrophotometer for the appropriate wavelength, measurements of the sample solutions are taken, and absorbance values are compared to determine the iron concentration in the water sample.
Q & A
What is a spectrophotometer?
-A spectrophotometer is a laboratory instrument used to analyze the composition and concentration of materials by measuring the amount of light they absorb or transmit.
How does a spectrophotometer work?
-The spectrophotometer uses a white light source that passes through a prism to decompose light into various colors of the visible spectrum. The light is then passed through a solution in a cuvette, where the device measures the absorbance and transmittance of light.
What happens to the light in a spectrophotometer?
-The light first passes through a prism, which splits it into different colors. A monochromator is then used to select a specific wavelength of light to analyze the sample. Some of the light is absorbed by the sample, and the rest is transmitted to the detector.
What is the role of the monochromator in a spectrophotometer?
-The monochromator is used to select a specific wavelength or range of wavelengths from the spectrum of light to pass through the sample. This helps in isolating the specific light that interacts with the sample to obtain accurate readings.
What is absorbance and how is it measured?
-Absorbance refers to the amount of light absorbed by the sample. It is measured by the spectrophotometer, which calculates the difference between the light that passes through the sample and the light that reaches the detector.
What is transmittance in a spectrophotometer?
-Transmittance is the amount of light that passes through the sample and reaches the detector. It is inversely related to absorbance, as more light transmitted means less light absorbed by the sample.
How can a spectrophotometer help determine the concentration of solutes?
-By measuring the absorbance or transmittance of a sample, the spectrophotometer allows comparison with known concentrations of solutes, enabling the calculation of the concentration of solutes in the unknown sample.
What types of analyses can be performed using a spectrophotometer?
-A spectrophotometer can be used for various analyses, including environmental water testing for organic and inorganic compounds, such as pesticides and heavy metals.
How is a spectrophotometer used to determine the amount of iron in water?
-To determine iron in water, a reagent is added to the sample that reacts with dissolved iron, turning the solution a specific color. The spectrophotometer then measures the absorbance of the colored solution, allowing the calculation of the iron concentration.
Why are standard solutions with known concentrations used in spectrophotometry?
-Standard solutions with known concentrations are used to create a calibration curve. This allows the spectrophotometer to compare the absorbance of the sample to the known values, making it possible to determine the unknown concentration of the substance in the sample.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة

Colorimetry

Spektrofotometer UV Vis Double Beam - Pengertian, Fungsi, Jenis-Jenis dan Bagian-Bagian

SPEKTROFOTOMETER UV-VIS THERMO-GENESYS150 | FARMASI ANALISIS - STFI Bandung
Working Principle of UV Spectrophotometer

Using a spectrophotometer

SPEKTROFOTOMETRI UV-Vis | Prinsip Kerja Spektrofotometri UV-Vis
5.0 / 5 (0 votes)
