IMPORTANCIA DEL AGUA COMO DISOLVENTE
Summary
TLDRIn this video, Miss Marce explores the vital role of water as a universal solvent. She explains how water, making up 75% of the human body at birth, is essential for biological processes. Water’s unique ability to dissolve a wide variety of substances through hydrogen bonding makes it an ideal solvent, though it cannot dissolve non-polar substances like oils and fats. The video also touches on solubility, the impact of dissolved substances on water's properties, and the importance of water in industries. Additionally, it addresses the issue of water pollution, specifically focusing on wastewaters from domestic, industrial, and agricultural sources.
Takeaways
- 😀 Water is a fundamental element for life on Earth, supporting biological processes that sustain life.
- 😀 The human body contains about 75% water at birth, making it essential for various bodily functions.
- 😀 Despite having no color, taste, or odor, water is the most powerful solvent, capable of dissolving many substances.
- 😀 Water is called a universal solvent because of its ability to form hydrogen bonds with various substances.
- 😀 The concept of solubility refers to the ability of a substance to dissolve in another, like sugar dissolving in water.
- 😀 Water cannot dissolve non-polar substances like fats and oils, which do not interact with water molecules.
- 😀 A solvent, like water, is a substance that dissolves a solute, such as sugar, creating a homogeneous solution.
- 😀 Some polar solutes that water can dissolve include salts, sugars, acids, and gases like oxygen and carbon dioxide.
- 😀 The addition of substances like salt to water can change its physical properties, such as increasing its density and boiling point.
- 😀 Water is widely used in industries, including in products like detergents, medicines, and food items, but contamination in natural water sources must be considered.
- 😀 Wastewater, or residual water, can be classified into three types: domestic, industrial, and agricultural, each with its specific contaminants.
Q & A
Why is water considered a universal solvent?
-Water is considered a universal solvent because it can dissolve a wide variety of substances due to its ability to form hydrogen bonds with other molecules, particularly polar substances.
How much of the human body is made up of water?
-At birth, the human body is composed of about 75% water, which is essential for various biological processes.
What is solubility and how does it relate to water?
-Solubility is the ability of a substance (solute) to dissolve in another substance (solvent). In the case of water, it can dissolve many polar solutes due to its polar nature, but it does not dissolve non-polar substances like oils and fats.
Why doesn’t water dissolve oils and fats?
-Water cannot dissolve oils and fats because these substances are non-polar and lack the partial positive and negative charges that allow them to interact with water molecules.
What happens when salt is added to water?
-When salt is added to water, the water's density increases and its boiling point rises due to the dissolved salt molecules.
What is the definition of a solvent?
-A solvent is a substance, usually present in greater quantity, in which a solute dissolves to form a solution. In the case of water, it acts as a solvent for various solutes like sugar and salt.
What are the three types of wastewater?
-The three types of wastewater are domestic or urban wastewater, industrial wastewater, and agricultural wastewater. Each type comes from different sources, such as homes, industries, and agricultural activities.
What makes water a vital element for life on Earth?
-Water is essential for life on Earth because it supports biological processes necessary for the growth, reproduction, and survival of living organisms, including humans.
What is the impact of water pollution on the environment?
-Water pollution, caused by contaminants like chemicals and waste products, can negatively affect ecosystems, human health, and the availability of clean water for various purposes.
How is water used in the industrial sector?
-In the industrial sector, water is used as a solvent in the production of products like detergents, medicines, and foods. It also plays a role in cooling systems, cleaning, and other processes.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
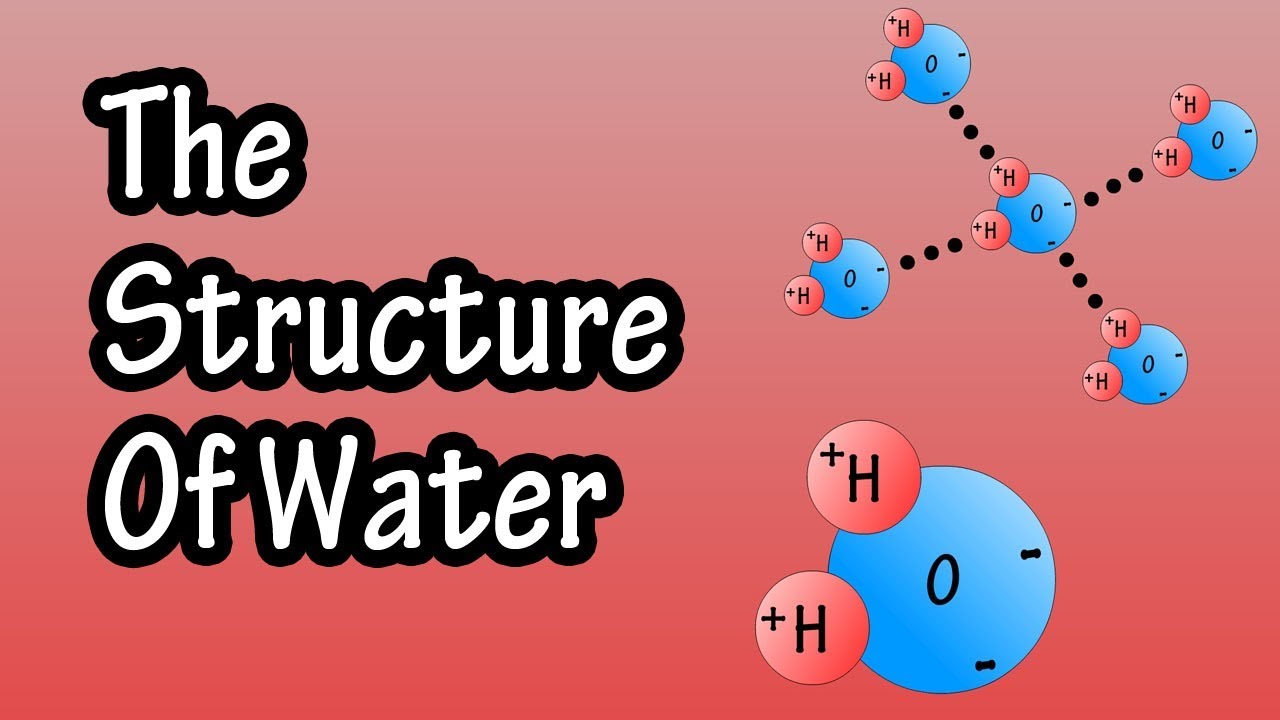
Structure Of Water Molecule - Chemistry Of Water - Properties Of Water - Composition Of Water

IB Biology A1.1

Água e Eletrólitos - Equilíbrio Hidroeletrolítico - Bases da Nutrição 15

IB Biology 2.2 - Water - Interactive Lecture

El Agua - Propiedades Físicas y Químicas - Importancia Biológica

Hydrogen bonding in water | Water, acids, and bases | Biology | Khan Academy
5.0 / 5 (0 votes)
