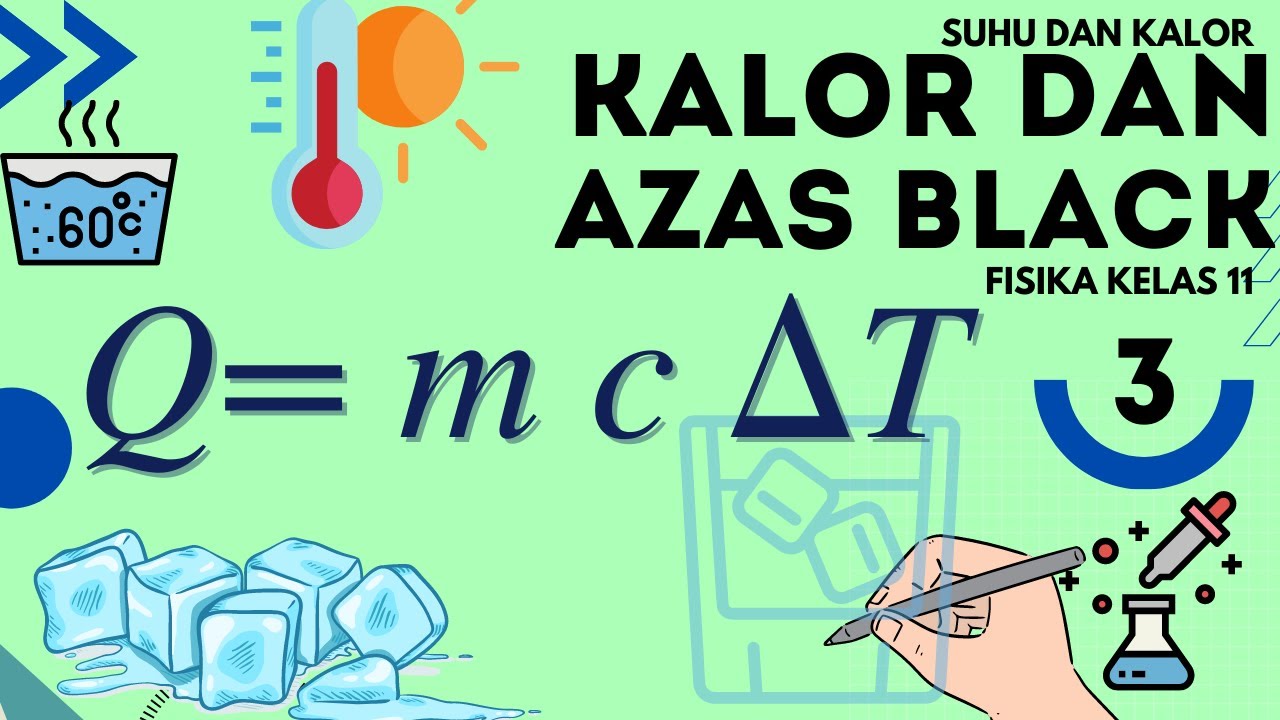FISIKA KELAS XI | SUHU DAN KALOR (PART 2) - KALOR DAN PENGARUHNYA
Summary
TLDRIn this video, Yusuf Ahmad explains the concept of heat (kalor) in physics, focusing on its effects on temperature and phase changes. The lesson covers key topics such as how heat flows from hotter to cooler objects, the formulas for calculating heat, and the different phase transitions (like melting, boiling, and sublimation). Yusuf also provides practical examples, demonstrating calculations using the formulas Q = mcΔT and Q = mL for specific heat and latent heat. The video is designed for Class 11 physics students and aims to clarify how heat affects substances in various ways.
Takeaways
- 😀 Calor is the transfer of thermal energy from a hotter object to a cooler one.
- 😀 The formula for calculating heat required to change temperature is Q = MCΔt, where Q is heat in Joules or calories, M is mass, C is specific heat, and Δt is the temperature change.
- 😀 1 calorie is equivalent to 4.2 Joules, and the specific heat capacity of water is 4200 J/kg°C or 1 cal/g°C.
- 😀 Heat can cause three types of changes in matter: changes in temperature, state, and volume (expansion).
- 😀 The heat required to change the state of a substance (e.g., melting or evaporating) follows different formulas, like Q = mL for melting or Q = mU for vaporization, where L and U are latent heat of fusion and vaporization, respectively.
- 😀 A graphical representation of phase changes and temperature changes shows different behaviors of matter at different stages, such as from solid to liquid (melting) and from liquid to gas (evaporation).
- 😀 When temperature changes occur, there is no phase change, and when a phase change occurs, the temperature remains constant until the change is complete.
- 😀 Example: For 100 grams of water heated from 20°C to 80°C, the heat required can be calculated using the formula Q = MCΔt, with appropriate unit conversions.
- 😀 Example: To melt 250 grams of ice at -50°C, you must first heat the ice from -50°C to 0°C and then melt it. The total heat required is the sum of the heat needed for temperature change and the heat for the phase change.
- 😀 The heat calculation steps for both water and ice examples illustrate the application of calorimetry in everyday thermal processes, and the values are expressed both in calories and Joules.
- 😀 The content emphasizes the importance of understanding calorimetry formulas for solving real-life physics problems related to heat transfer, temperature changes, and phase transitions.
Q & A
What is heat (kalor) and how does it flow?
-Heat is the form of energy that flows from a body at a higher temperature to a body at a lower temperature. It can cause changes in the temperature, state, and size (thermal expansion) of materials.
What is the formula to calculate heat required to change the temperature of a substance?
-The formula is Q = mcΔT, where Q is the heat in joules or calories, m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature.
What is the relationship between calories and joules?
-1 calorie is equivalent to 4.2 joules.
What is specific heat capacity and how is it used in calorimetry?
-Specific heat capacity (c) is the amount of heat needed to raise the temperature of 1 kg of a substance by 1°C. It is used in calorimetry to calculate the heat required for temperature changes.
What happens when heat causes a change in the state of a substance?
-When heat causes a change in the state of a substance, the formula Q = mL is used for processes like melting or boiling, where L is the latent heat of fusion or vaporization, respectively.
What are the different types of state changes that heat can cause?
-Heat can cause various state changes such as melting (solid to liquid), boiling (liquid to gas), freezing (liquid to solid), condensation (gas to liquid), sublimation (solid to gas), and deposition (gas to solid).
What does the graph of temperature and state change illustrate?
-The graph shows temperature changes in relation to phase transitions. For example, it can show the heat required to raise the temperature of ice to its melting point, to melt it into water, and then to boil the water into steam.
How is heat calculated during a phase change, like melting or boiling?
-During a phase change, heat is calculated using Q = mL, where m is the mass and L is the latent heat. This formula applies during melting (fusion) and boiling (vaporization).
How do you calculate the total heat required for multiple temperature and state changes?
-To calculate the total heat, sum the heat required for each individual step. For example, heating ice, melting it, heating water, and then boiling it involves calculating the heat for each of these steps separately and adding them together.
What is the heat required to raise the temperature of 100g of water from 20°C to 80°C?
-The heat required can be calculated using the formula Q = mcΔT. For 100g of water, c = 1 cal/g°C, and ΔT = 80°C - 20°C = 60°C. Therefore, Q = 100 * 1 * 60 = 6000 calories, or 6000 * 4.2 = 25200 joules.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
5.0 / 5 (0 votes)