Pengaruh Penambahan Konsentrasi terhadap Pergeseran Kesetimbangan
Summary
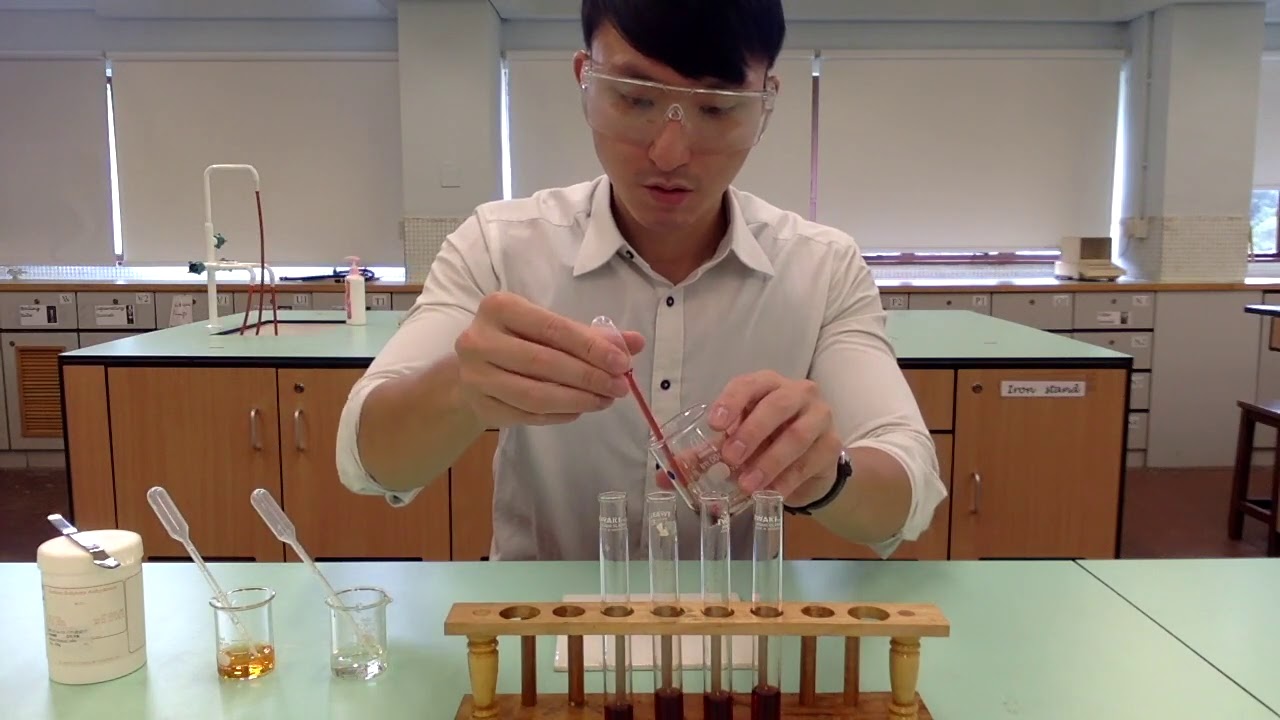
TLDRThis chemistry experiment video demonstrates how concentration affects chemical equilibrium. The experiment involves cobalt chloride solution (CoCl₂), and by adding concentrated hydrochloric acid (HCl) or water, the equilibrium shifts. Adding HCl makes the solution darker, indicating a shift towards cobalt(III) complexes, while adding water lightens the solution, showing a shift towards cobalt(II) ions. The video emphasizes the dynamic nature of equilibrium and how changes in concentration influence the reaction, making it a visual and practical exploration of equilibrium principles in chemistry.
Takeaways
- 😀 The video introduces a chemistry experiment related to equilibrium shifts, explaining the concept of dynamic equilibrium in chemical reactions.
- 😀 Chemical equilibrium refers to a state where the rate of the forward reaction is equal to the rate of the reverse reaction, maintaining a balance between products and reactants.
- 😀 Equilibrium can be influenced by three main factors: concentration, temperature, and pressure, which can cause a shift in the equilibrium position.
- 😀 The experiment in the video demonstrates the effect of concentration changes on equilibrium shifts.
- 😀 The first part of the experiment involves dissolving cobalt(II) chloride (CoCl2) crystals into a 1M concentration solution using distilled water.
- 😀 The experiment uses concentrated hydrochloric acid (HCl) with a concentration of approximately 12M, requiring the use of a fume hood for safety.
- 😀 In the experiment, the effect of adding HCl versus water to the cobalt chloride solution is observed to see how they impact the equilibrium position.
- 😀 Adding concentrated HCl to the cobalt chloride solution causes the color to darken, shifting the equilibrium toward the formation of a cobalt complex ion.
- 😀 When water is added to the cobalt chloride solution, the color becomes lighter or fades, indicating the equilibrium shifts to the left, favoring the formation of Co2+ ions.
- 😀 The video concludes with the observation that adding water shifts the equilibrium toward the left (forming more Co2+ ions), while adding HCl shifts it to the right (forming a cobalt complex).
Q & A
What is chemical equilibrium?
-Chemical equilibrium refers to the state in a chemical reaction where the rates of the forward and reverse reactions are equal, resulting in no net change in the concentrations of reactants and products.
What is meant by dynamic equilibrium?
-Dynamic equilibrium refers to a state in a chemical reaction where the reactants and products are constantly interconverting at equal rates, although there is no visible change in the concentration of substances.
What factors influence chemical equilibrium?
-The three main factors that influence chemical equilibrium are concentration, temperature, and pressure.
What is the purpose of shifting equilibrium in an experiment?
-The purpose of shifting equilibrium in an experiment is to observe how the reaction adjusts to reach a new equilibrium state, where the forward and reverse reaction rates are once again balanced.
What substances are used in the experiment described in the transcript?
-In the experiment, cobalt chloride (CoCl2) and hydrochloric acid (HCl) are used to study the effect of concentration changes on the equilibrium.
How does the addition of hydrochloric acid affect the equilibrium?
-Adding hydrochloric acid to the cobalt chloride solution shifts the equilibrium to the right, forming a blue complex ion, indicating that the reaction favors the production of products.
What happens when water is added to the cobalt chloride solution?
-When water is added to the cobalt chloride solution, the equilibrium shifts to the left, causing the color to fade and become pink, as the reaction favors the formation of reactants.
Why is the experiment conducted in a fume hood?
-The experiment is conducted in a fume hood because hydrochloric acid (HCl) is a concentrated acid, which can be hazardous, and the fumes need to be safely vented out.
What is indicated by the color change in the experiment?
-The color change in the experiment indicates the direction of the equilibrium shift. A blue color indicates a shift to the right (formation of products), while a pink color indicates a shift to the left (formation of reactants).
How does the concentration of HCl affect the equilibrium in this experiment?
-Increasing the concentration of HCl shifts the equilibrium to the right, as evidenced by the color changing to blue, indicating more complex ions are formed.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة

Praktikum Pergeseran Kesetimbangan Kimia

Kimia - ANIMASI PERCOBAAN PENGARUH KONSENTRASI DAN VOLUME PADA PERGESERAN KESETIMBANGAN

PRAKTIKUM PERGESERAN KESETIMBANGAN
Experiment 41.1 Effect of changing concentration on equilibrium position

Praktikum Faktor Kesetimbangan Kimia MGMP KIMIA PENABUR Jakarta

Kimia Dasar 2 : Kesetimbangan (Spektrofotometer UV-VIS)
5.0 / 5 (0 votes)
