9.2 Ideal Stoichiometric Calculations
Summary
TLDRThis video explains ideal stoichiometric calculations in chemistry, focusing on various examples to demonstrate the process. The presenter breaks down how to convert between moles and grams using molar ratios derived from chemical equations, while emphasizing the importance of canceling units for accurate results. The video covers simple to complex calculations, including converting molar amounts between substances and mass-to-mass conversions. Through examples like hydrogen combustion and tin fluoride synthesis, viewers are guided through the essential steps in stoichiometry, with a strong focus on dimensional analysis and systematic unit cancellation for correct answers.
Takeaways
- 😀 Stoichiometric calculations model chemical reactions under ideal conditions, where all reactants react completely.
- 😀 In real-world reactions, some reactants may not completely react or may remain uncombined, but stoichiometry assumes ideal reactions.
- 😀 Stoichiometric calculations are based on molar ratios between reactants and products, using coefficients from chemical equations.
- 😀 For simple problems, you can convert moles of one substance to moles of another using the mole ratio from the balanced equation.
- 😀 When converting from moles to grams, use the molar mass of the substance to complete the calculation.
- 😀 Dimensional analysis (canceling units) is crucial in stoichiometric calculations to ensure correct conversions and results.
- 😀 More complex problems involve converting from mass to moles, then using molar ratios to find other masses or amounts.
- 😀 The stoichiometric process for mass-to-mass problems includes converting the given mass to moles, then to the unknown mass using the mole ratio.
- 😀 For mole-to-mass conversions, the process involves calculating moles from mass and then using the mole ratio and molar mass to find the final mass.
- 😀 Consistently canceling out units at each step helps ensure that the correct units remain and the desired quantity is calculated correctly.
- 😀 Example problems involve using balanced chemical equations (e.g., hydrogen and water combustion or tin fluoride formation) to apply stoichiometric principles and find the desired quantities.
Q & A
What are ideal stoichiometric calculations based on?
-Ideal stoichiometric calculations are based on the assumption that all reactants completely react to form products, with no leftover reactants or incomplete reactions.
Why are real-world chemical reactions not ideal?
-In real-world reactions, some reactants may not fully react or may remain unreacted in the mixture due to incomplete bonding or other factors, unlike ideal conditions where full conversion is assumed.
What is the first step in solving stoichiometric problems involving moles of a substance?
-The first step is to identify the molar ratio between the given compound and the compound you're trying to find, based on the coefficients in the balanced chemical equation.
How do you convert moles of one substance to moles of another substance?
-You use the molar ratio derived from the coefficients in the balanced chemical equation to cancel out the units of the initial substance and convert to the required substance.
How can stoichiometric calculations be done when converting moles to mass?
-After finding the moles of the desired substance, you multiply by its molar mass to convert it to grams. The molar mass is used as a conversion factor to go from moles to mass.
In the combustion of hydrogen to form water, how do you find the grams of water produced?
-Start by determining the moles of hydrogen, then use the molar ratio to find moles of water produced. Multiply by the molar mass of water to convert moles to grams.
What is the role of dimensional analysis in stoichiometric calculations?
-Dimensional analysis helps ensure that units cancel out correctly at each step of the calculation, allowing you to convert from one quantity to another without confusion.
When given a mass of a substance, what intermediate step is necessary in stoichiometric calculations?
-You first convert the given mass to moles using the molar mass of the substance, which serves as an intermediate step before using the molar ratio to convert to the moles or mass of another substance.
How do you handle more complex stoichiometric problems where both mass and moles are involved?
-In complex problems, you convert the given mass to moles, use the molar ratio to find the moles of another substance, and then convert those moles to mass using the molar mass of the desired substance.
In the example of tin reacting with hydrofluoric acid, how do you calculate the mass of tin fluoride produced?
-You start with the mass of hydrofluoric acid, convert it to moles, use the molar ratio to find moles of tin fluoride, and finally convert moles of tin fluoride to grams using its molar mass.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
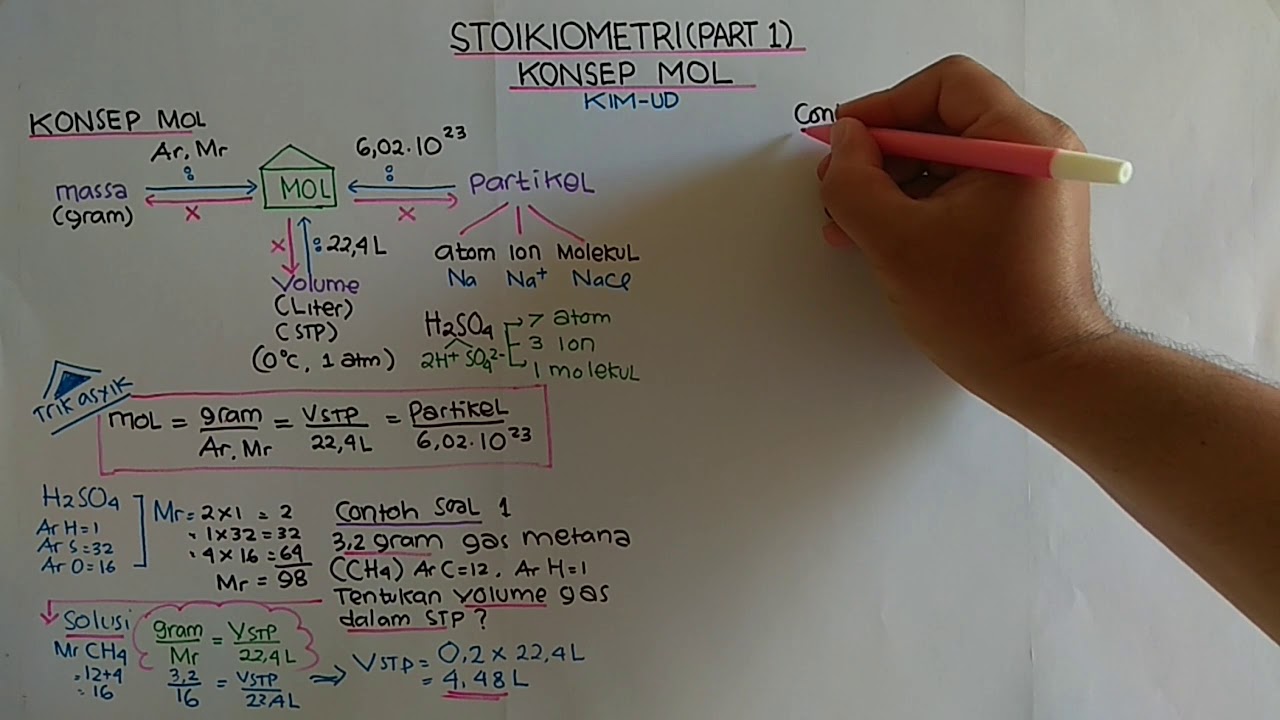
STOIKIOMETRI (PART 1) KONSEP MOL
Stoikiometri 1 (mol adalah jumlah)

Stoikiometri (3) | Menentukan Rumus Empiris Dan Rumus Molekul | Kimia Kelas 10

Stoffumsatz berechnen, Stöchiometrisches Rechnen | Chemie Endlich Verstehen

STOIKIOMETRI SINTESIS BIOETANOL DARI SUKROSA

Stoikiometri (5) | Perhitungan Dalam Persamaan Reaksi | Kimia Kelas 10
5.0 / 5 (0 votes)
