Resolução exercício
Summary
TLDRThis video explains the energy required to remove an electron from a hydrogen atom, starting from its ground state to infinity. It involves calculating the energy change using a formula that incorporates Planck's constant, the speed of light, and the Rydberg constant. The process is demonstrated step by step, with a specific example where the energy is converted from joules to electron volts. The video also touches on calculating the energy for a mole of hydrogen atoms, providing a thorough understanding of atomic energy transitions and the related mathematical concepts.
Takeaways
- 😀 The exercise is about calculating the energy required to remove an electron from a hydrogen atom, specifically from the n=1 energy level to infinity.
- 😀 The energy variation formula is introduced: ΔE = h * c * R_H * (1/n_i² - 1/n_f²), where h is Planck's constant, c is the speed of light, R_H is the Rydberg constant, and n_i and n_f are the initial and final energy levels.
- 😀 The initial energy level is n=1, and the final energy level is considered to be infinity, which makes 1/n_f² equal to zero.
- 😀 The calculation involves substituting values for Planck's constant (6.626 x 10^-34 J·s), speed of light (3 x 10^8 m/s), and the Rydberg constant (1.097 x 10^7 m^-1) into the equation.
- 😀 The energy variation for removing an electron from the hydrogen atom is calculated to be approximately 2.2 x 10^-18 joules.
- 😀 To convert this energy into electron volts (eV), the energy is divided by the conversion factor (1 eV = 1.602 x 10^-19 joules), resulting in 13.70 eV.
- 😀 This amount of energy (13.70 eV) corresponds to the energy required to remove one electron from a single hydrogen atom.
- 😀 The process can be extended to calculate the energy for a mole of hydrogen atoms, using Avogadro's number (6.02 x 10^23 atoms/mol).
- 😀 When calculating for a mole of hydrogen atoms, the energy required is approximately 1312 kJ/mol.
- 😀 The script also emphasizes that these calculations can be applied to electrons at higher energy levels, such as n=2, n=3, and beyond, not just n=1.
Q & A
What is the purpose of the exercise in the video script?
-The exercise aims to calculate the energy required to remove an electron from the hydrogen atom, starting from the first energy level (n=1) and moving to an infinite energy level.
What equation is used to calculate the energy required to remove an electron from a hydrogen atom?
-The equation used is ΔE = h × c × R∞ × (1/n₁² - 1/n₂²), where ΔE is the energy variation, h is Planck's constant, c is the speed of light, R∞ is the Rydberg constant, and n₁ and n₂ are the initial and final energy levels, respectively.
What values are used for the constants in the energy calculation?
-The values used are: Planck's constant (h) = 6.626 × 10⁻³⁴ J·s, speed of light (c) = 3 × 10⁸ m/s, and the Rydberg constant (R∞) = 1.097 × 10⁷ m⁻¹.
Why does 1/n² become zero when n₂ is infinite?
-When n₂ approaches infinity, the term 1/n₂² approaches zero, meaning the final energy level is considered to be infinitely far from the nucleus, effectively removing the electron from the atom.
What is the calculated energy required to remove an electron from a hydrogen atom?
-The calculated energy required to remove the electron from the hydrogen atom is approximately 2.2 × 10⁻¹⁸ joules.
How is the energy in joules converted to electron volts (eV)?
-To convert joules to electron volts, the energy in joules is divided by the value of 1 electron volt, which is 1.602 × 10⁻¹⁹ joules. For this case, the energy of 2.2 × 10⁻¹⁸ joules corresponds to about 13.7 eV.
What does the term 'electron volts' represent in this context?
-Electron volts (eV) represent a unit of energy commonly used in atomic and particle physics, where 1 eV is the energy gained by an electron moving through a potential difference of 1 volt.
How is the energy required to remove an electron from a mole of hydrogen atoms calculated?
-To calculate the energy required for a mole of hydrogen atoms, the energy calculated for one atom (2.2 × 10⁻¹⁸ joules) is multiplied by Avogadro's constant (6.02 × 10²³ atoms/mol), resulting in an energy of approximately 1.3 × 10⁶ joules per mole.
What is the final energy value for one mole of hydrogen atoms in kilojoules?
-The energy required to remove the electron from one mole of hydrogen atoms is approximately 1.312 × 10³ kilojoules per mole.
Can the same method be used to calculate the energy for electrons in higher energy levels?
-Yes, the same method can be applied to calculate the energy required to remove electrons from higher energy levels, such as n=2, n=3, and so on. The only change is in the values of n₁ and n₂ in the equation.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
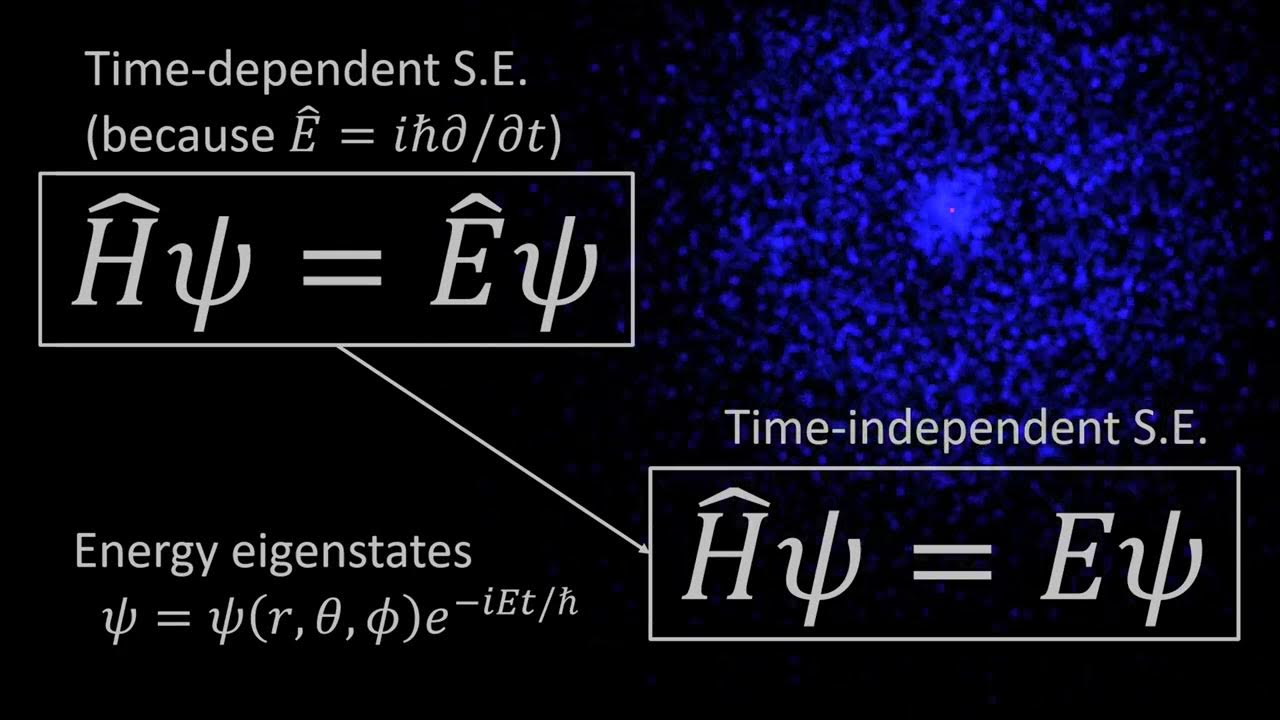
The Hydrogen Atom, Part 1 of 3: Intro to Quantum Physics

AQA A-Level Chemistry - Ionisation Energies

Bohr Model in Brief: The planetary model, its connection to emission spectra & quantized electrons.
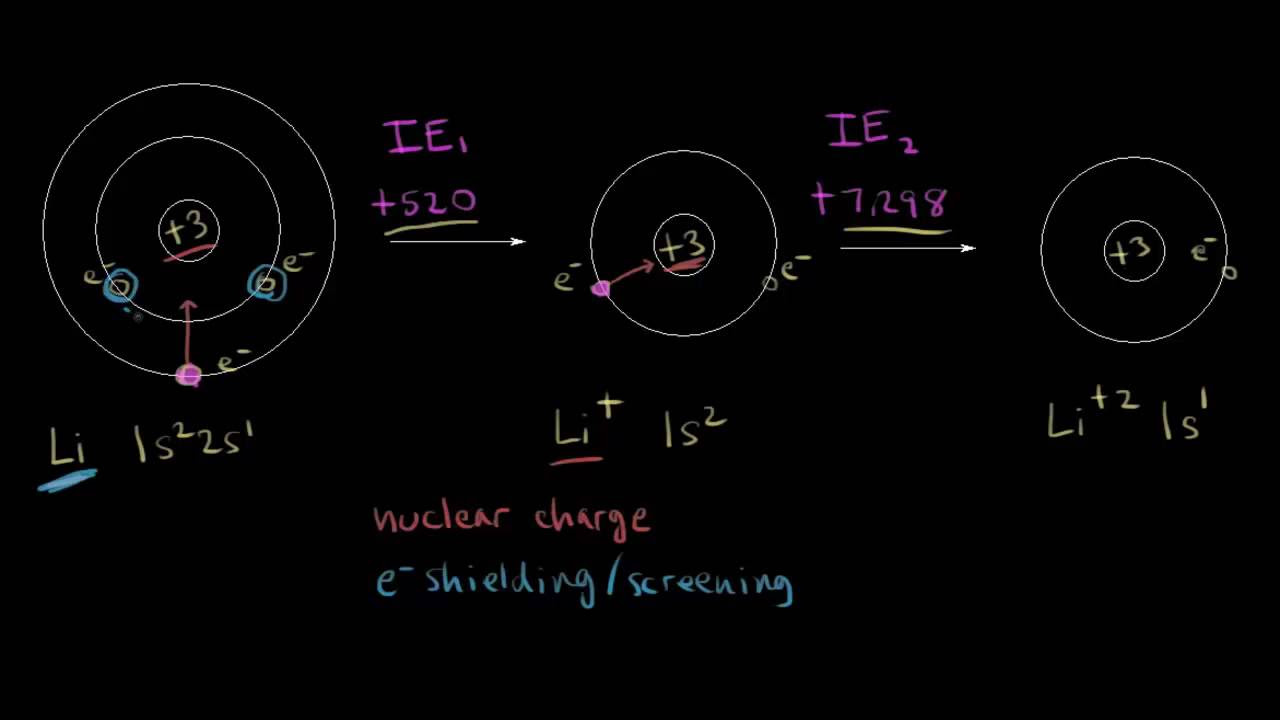
First and second ionization energy | Atomic structure and properties | AP Chemistry | Khan Academy

Electron Configuration of Atoms + Shortcut Tutorial Video
Coulomb's Law
5.0 / 5 (0 votes)
