Química Orgânica II – Aula 01 – Aldeídos e cetonas – estrutura e propriedades
Summary
TLDRThis educational lecture delves into aldehydes and ketones, focusing on their structural features, properties, and reactivity. The carbonyl group (C=O) is highlighted as the key functional feature, with discussions on resonance structures and how electron delocalization impacts molecular reactivity. The lecture also covers the influence of molecular size on boiling points, hydrogen bonding, and dipole interactions. Additionally, it explores the nomenclature of these compounds, explaining how they are named based on the parent alkane structure. The session sets the stage for further exploration into the practical applications of aldehydes and ketones in flavor and fragrance industries.
Takeaways
- 😀 The course is starting the 14th semester of Organic Chemistry II, building on concepts from Organic Chemistry I.
- 😀 The main focus is on new organic functions, starting with aldehydes and ketones, and their structural and property-related characteristics.
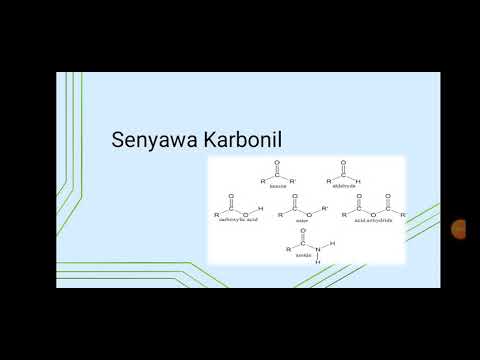
- 😀 The key functional group for aldehydes and ketones is the carbonyl group (C=O), which determines their properties and reactivity.
- 😀 Resonance structures are used to represent the delocalization of electrons in aldehydes and ketones, contributing to their molecular behavior.
- 😀 Aldehydes and ketones are reactive because of the partial positive charge on the carbonyl carbon, which attracts nucleophiles in reactions.
- 😀 The lecture explains that resonance structures are not in equilibrium with one another; they are models to understand reactivity and molecular properties.
- 😀 Natural examples of aldehydes and ketones include vanillin (from vanilla), cinnamaldehyde (from cinnamon), and carvone (from mint).
- 😀 The differences in molecular structures, such as alkyl groups attached to the carbonyl group, affect the properties and reactivity of aldehydes and ketones.
- 😀 The naming of aldehydes and ketones follows systematic nomenclature, with common names like acetone for propanone.
- 😀 Intermolecular forces, such as hydrogen bonds and dipole-dipole interactions, influence the boiling points and solubility of aldehydes and ketones.
- 😀 The boiling points of aldehydes and ketones are higher for larger molecules due to stronger intermolecular forces, especially as chain length increases.
Q & A
What is the central functional group in aldehydes and ketones?
-The central functional group in both aldehydes and ketones is the carbonyl group (C=O), where a carbon atom is double-bonded to an oxygen atom.
How do resonance structures affect the reactivity of aldehydes and ketones?
-Resonance structures indicate that the carbonyl carbon in aldehydes and ketones carries a partial positive charge, making it electrophilic and susceptible to nucleophilic attack. The delocalization of electrons to the oxygen atom contributes to the reactivity of these compounds.
Why are aldehydes generally more reactive than ketones?
-Aldehydes are more reactive than ketones because the carbonyl carbon in aldehydes is less shielded by alkyl groups (it has only one alkyl group and a hydrogen), making it more susceptible to nucleophilic attack compared to ketones, which have two alkyl groups attached.
What is the rule for naming aldehydes and ketones in organic chemistry?
-Aldehydes are named by replacing the '-e' ending of the parent hydrocarbon with '-al', while ketones are named by replacing '-e' with '-one'. For example, methanal for formaldehyde and propanone for acetone.
Can you give examples of aldehydes and ketones found in nature?
-Yes, examples of aldehydes and ketones found in nature include vanillin (from vanilla), cinnamaldehyde (from cinnamon), carvone (from mint), and cuminaldehyde (from cumin seeds). These compounds contribute to the characteristic flavors and aromas of these plants.
How do the boiling points of aldehydes and ketones change with molecular size?
-As the molecular size of aldehydes and ketones increases, their boiling points also increase due to stronger intermolecular forces such as London dispersion forces. Larger molecules have more points of interaction, leading to higher boiling points.
What role does hydrogen bonding play in the physical properties of aldehydes and ketones?
-Hydrogen bonding plays a significant role in determining the physical properties of aldehydes and ketones. Although aldehydes and ketones cannot form hydrogen bonds as strongly as alcohols, they can engage in dipole-dipole interactions, which influence properties like boiling point and solubility.
Why do aldehydes generally have lower boiling points than ketones of similar size?
-Aldehydes tend to have lower boiling points than ketones of similar size because the dipole-dipole interactions in ketones are generally stronger due to the presence of two alkyl groups attached to the carbonyl carbon, compared to just one alkyl group in aldehydes.
What is the importance of understanding resonance structures in aldehydes and ketones?
-Understanding resonance structures is crucial because they explain the distribution of electrons in the molecule, helping us understand the electrophilic nature of the carbonyl carbon and how it influences the reactivity of aldehydes and ketones in chemical reactions.
How do intermolecular forces influence the physical properties of aldehydes and ketones?
-Intermolecular forces such as hydrogen bonding, dipole-dipole interactions, and London dispersion forces play a key role in the physical properties of aldehydes and ketones. These forces impact boiling points, solubility, and melting points, with larger molecules typically having higher boiling points due to more points of interaction.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآن5.0 / 5 (0 votes)