How to Ligate and Transform DNA Fragments
Summary
TLDRThis video outlines a step-by-step protocol for performing ligation and transformation, specifically focusing on inserting a red fluorescent protein (RFP) gene into a vector. It covers the key steps of calculating reagent volumes, preparing control samples, incubating ligation reactions, and transforming competent cells. The process also includes important tips on increasing transformation efficiency and verifying successful results. The video emphasizes the importance of controls, proper handling of reagents, and correct incubation times to ensure optimal success in molecular cloning experiments.
Takeaways
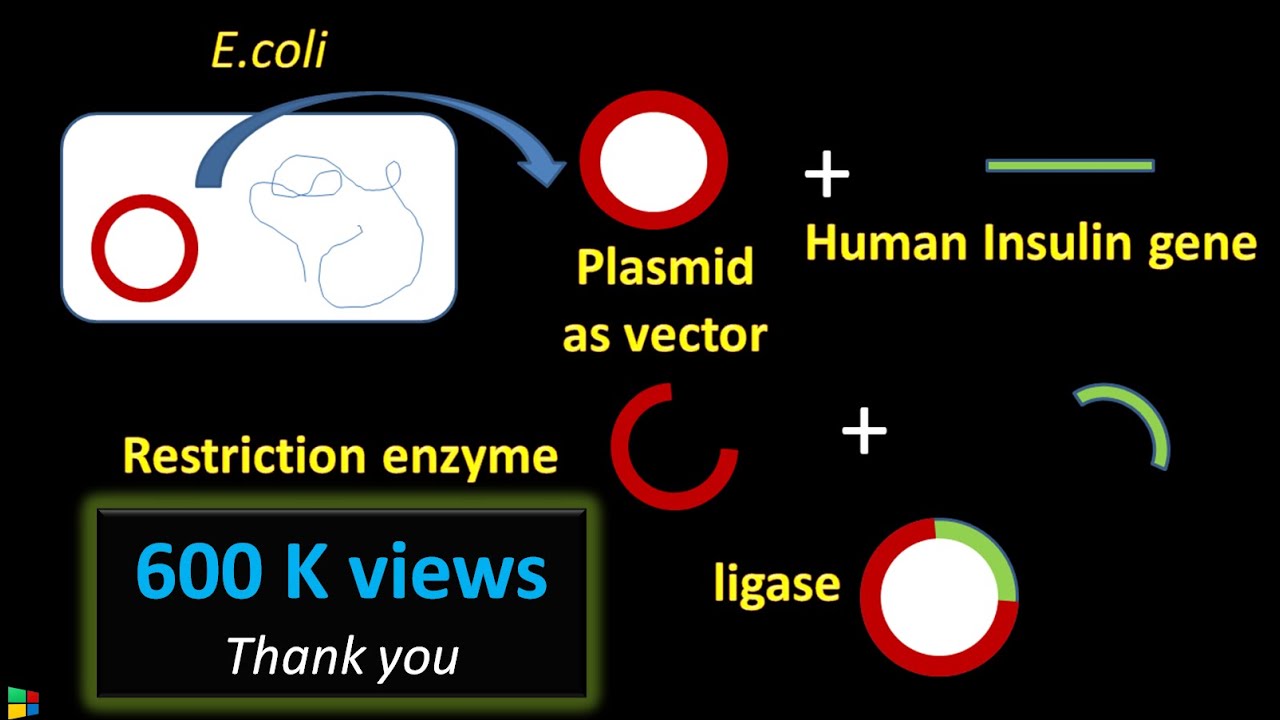
- 😀 The ligation and transformation protocol involves inserting the RFP gene into a vector using a 1:3 molar ratio of vector to insert.
- 😀 The total volume for the ligation reaction is 10 µL, with components added in precise amounts for optimal results.
- 😀 A negative control is essential for verifying that the colonies are a result of the ligation, not residual vector.
- 😀 The ligation mix includes vector, insert, ligation buffer, water, and ligase enzyme, added in that order to maintain enzyme activity.
- 😀 After preparing the ligation mix, it’s incubated at 16°C overnight or at room temperature for 1-2 hours for maximum efficiency.
- 😀 Competent cells are aliquoted into two tubes, one for the experimental sample and one for the negative control.
- 😀 The ligation mix is added to the competent cells, followed by a brief incubation on ice before performing a heat shock at 42°C for 30 seconds.
- 😀 After heat shock, recovery medium (LB or SOC) is added to help the cells recover, followed by 1 hour of incubation at 37°C with shaking.
- 😀 Transformed cells are plated on agar plates, with 100 µL of cells spread evenly using sterile beads for proper colony growth.
- 😀 Successful transformation is confirmed by the appearance of colonies on the experimental plate and the absence of colonies on the control plate.
Q & A
What is the purpose of the ligation and transformation protocol in this video?
-The purpose of the protocol is to ligate a DNA insert (RFP) into a vector and then transform the ligated vector into competent cells. The goal is to create recombinant bacteria that carry the desired genetic material.
Why is it important to maintain a 1:3 ratio of vector to insert during the ligation?
-The 1:3 ratio of vector to insert in terms of moles ensures optimal conditions for the ligase enzyme to ligate the DNA insert into the vector. A correct ratio increases the likelihood of successful ligation.
What is the role of the ligation buffer in this experiment?
-The ligation buffer contains ATP and magnesium chloride, which are essential for the ligase enzyme to function. ATP provides energy for the ligation reaction, and magnesium chloride acts as a cofactor for the enzyme.
Why is the ligase enzyme added last in the reaction mixture?
-The ligase enzyme is added last to the reaction mixture to prevent premature inactivation. If added too early, the enzyme might lose its efficiency due to exposure to other components before the reaction is ready.
What is the purpose of the negative control in the ligation protocol?
-The negative control contains only the vector and no insert. It is used to ensure that any colonies observed in the experimental sample are due to successful ligation and not background growth from the vector alone.
What is the recommended incubation time for the ligation reaction, and what is the reasoning behind it?
-The ligation reaction can be incubated overnight at 16°C for maximum efficiency, or for 1-2 hours at room temperature if time is limited. Overnight incubation allows for optimal ligation of the insert into the vector.
Why should the tubes be kept on ice after adding the ligation mix to competent cells?
-Keeping the tubes on ice helps to slow down any biological activity and stabilizes the competent cells before heat shock. It is an essential step for maximizing transformation efficiency.
What is the purpose of the heat shock step in the transformation protocol?
-The heat shock at 42°C for 30 seconds induces the competent cells to take up the plasmid DNA. This is a critical step in the transformation process, as it increases the permeability of the cell membrane to the DNA.
What type of medium is used for recovery after the heat shock, and why?
-After heat shock, cells are recovered in LB or SOC medium. SOC medium is recommended for higher transformation efficiency as it is richer in nutrients, helping the cells to recover more effectively and express the newly acquired genetic material.
How do you know if the ligation and transformation were successful?
-Success is determined by colony growth on the experimental plate. If colonies appear, it indicates that the ligation was successful and the insert was transformed into the competent cells. The negative control should show no colonies, confirming that the vector alone did not transform.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآن5.0 / 5 (0 votes)