PASHU PARICHAR GENERAL SCIENCE MCQ CLASS | भौतिक एवं रासायनिक परिवर्तन | Most Questions By DBM SIR
Summary
TLDRThe video explains the concepts of physical and chemical changes through detailed examples and MCQs. The speaker highlights key differences between these changes, such as the melting of wax being a physical change and the rusting of iron being a chemical change. The discussion includes the impact of energy transfer and the irreversible nature of chemical changes. The speaker aims to help viewers understand these concepts thoroughly, especially for exams, assuring them that with this knowledge, they can confidently answer related questions.
Takeaways
- 😀 A physical change involves a change in physical properties without altering the chemical composition of the substance.
- 😀 A chemical change results in the formation of new substances, with a change in the chemical composition of the original substance.
- 😀 Examples of physical changes include melting wax and bending a metal, where no new substance is formed.
- 😀 Examples of chemical changes include rusting of iron and burning of a candle, where new substances are formed.
- 😀 The melting of wax is considered a physical change because its chemical structure remains unchanged.
- 😀 The burning of a candle is a chemical change as it results in the creation of new substances, such as carbon dioxide and water.
- 😀 In the case of iron rusting, the chemical structure of the iron changes, leading to the formation of iron oxide.
- 😀 The distinction between physical and chemical changes is important for understanding various chemical processes.
- 😀 The speaker emphasizes that students should clearly understand the difference between physical and chemical changes to perform well in exams.
- 😀 A multiple-choice question example was used to highlight the definition and characteristics of a physical change, reinforcing the key concepts.
Q & A
What is the difference between physical and chemical changes as discussed in the script?
-Physical changes involve changes in physical properties like shape or state without altering the substance itself. Chemical changes result in the formation of new substances, with a change in chemical properties.
What is an example of a physical change provided in the script?
-An example of a physical change is the melting of wax. The wax changes from solid to liquid, but its chemical composition remains the same.
What is an example of a chemical change mentioned in the video?
-The rusting of iron is an example of a chemical change. In this process, iron reacts with oxygen to form rust, which is a new substance.
Why is the burning of a candle considered a chemical change?
-The burning of a candle is considered a chemical change because the wax undergoes combustion, resulting in the formation of new substances like carbon dioxide and water vapor.
What is the main characteristic of a physical change?
-In a physical change, only the physical properties of a substance are altered (e.g., state, shape) without any change in its chemical composition.
How does the speaker differentiate between physical and chemical changes in the context of energy transfer?
-The speaker mentions that in physical changes, energy transfer does not occur, while in chemical changes, there is a transfer of energy, often in the form of heat or light.
What is the role of energy in chemical changes according to the script?
-In chemical changes, energy is transferred as bonds are broken and formed, leading to the creation of new substances. This transfer of energy can be observed as heat, light, or sound.
What kind of change is described when the wax in a candle melts?
-The melting of the wax in a candle is described as a physical change because it only alters the state of the wax, from solid to liquid, without any chemical transformation.
Why does the speaker emphasize the importance of understanding physical and chemical changes for exams?
-The speaker emphasizes this understanding because knowledge of physical and chemical changes is fundamental for various competitive exams, and these concepts often appear in questions.
What was the speaker's overall message regarding physical and chemical changes?
-The speaker's overall message was to ensure that viewers understand the difference between physical and chemical changes, as it is crucial for answering related questions correctly in exams.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
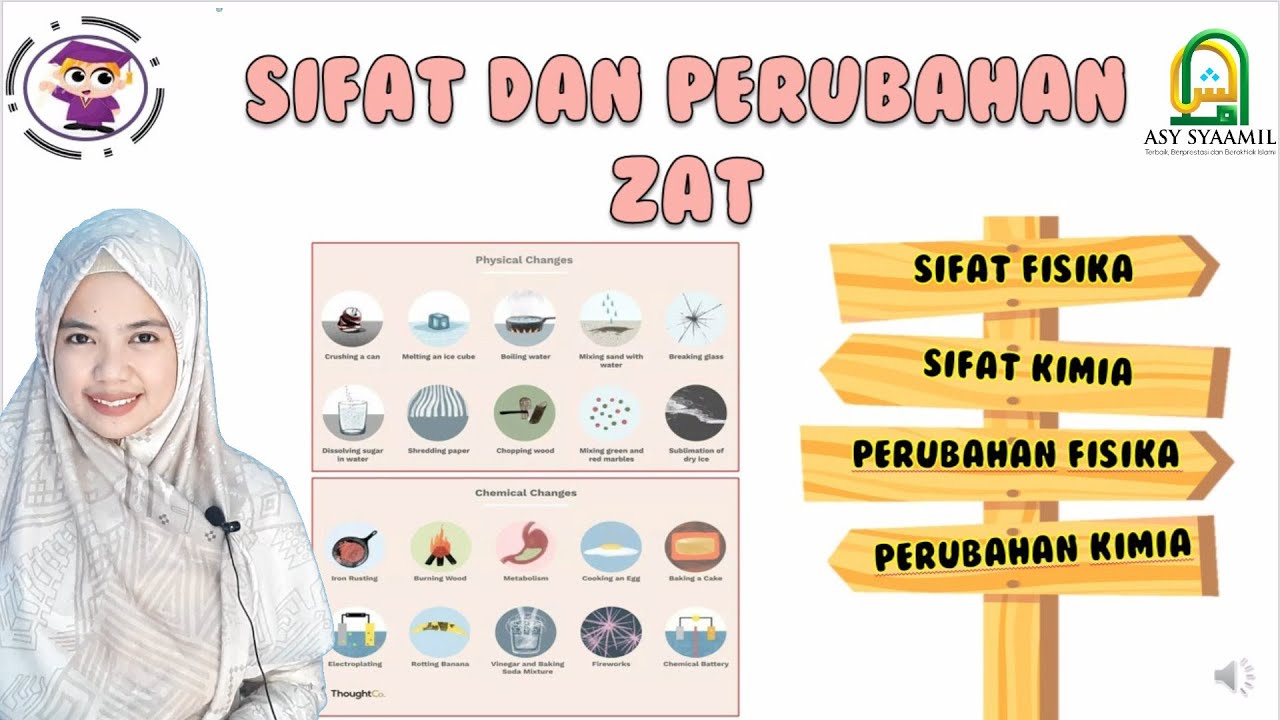
SIFAT DAN PERUBAHAN ZAT | SIFAT FISIKA DAN KIMIA | PERUBAHAN FISIKA DAN KIMIA

Como diferenciar transformações Físicas das Químicas |Mapa Mental|

Chemical and Physical Changes – Quiz Edition

Is Matter around us Pure in 20 Minutes🔥| Class 9th | Rapid Revision | Prashant Kirad

10th Science 1 | Chapter 3 | Chemical Reactions & Equations | Lecture 1 | Maharashtra Board |

Perubahan Materi / KIMIA kelas 10 kurikulum merdeka , perubahan fisika dan perubahan kimia
5.0 / 5 (0 votes)
