Cellular Respiration Part 3: The Electron Transport Chain and Oxidative Phosphorylation
Summary
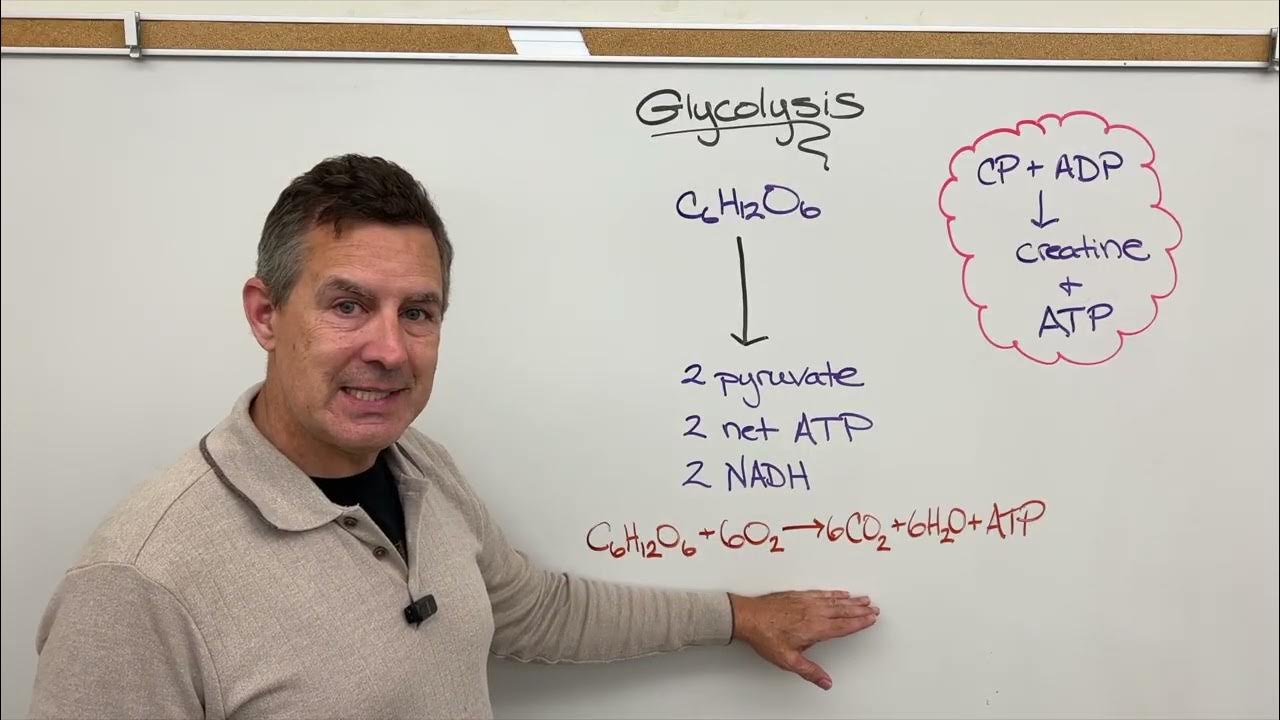
TLDRProfessor Dave explains cellular respiration, focusing on oxidative phosphorylation as the most efficient ATP generator. The process involves an electron transport chain with protein complexes I-IV and CoQ, creating a proton gradient that powers ATP synthase. From one glucose molecule, approximately 26-28 ATPs are produced. Mitochondria are hailed as the cell's engine, converting food into energy for cellular activities.
Takeaways
- 🔋 Oxidative phosphorylation is the most energy-rich pathway in cellular respiration.
- 🌀 Glycolysis and the citric acid cycle are the first two steps of cellular respiration but yield less energy compared to oxidative phosphorylation.
- 🚀 Electron transport chain (ETC) is a series of protein complexes in the mitochondrial membrane that facilitate redox reactions.
- 🛠️ Protein complexes I-IV and Coenzyme Q (CoQ) are key components of the ETC.
- ⚡ Electrons from NADH and FADH2 are fed into the ETC, generating a proton gradient across the mitochondrial membrane.
- 💧 The proton gradient drives ATP synthase, which synthesizes ATP through a process called chemiosmosis.
- 🌀 ATP synthase has a rotor-like structure that spins to catalyze the phosphorylation of ADP to ATP.
- 🍬 From a single glucose molecule, around 26-28 ATPs can be generated through oxidative phosphorylation.
- 🔄 Other food sources like proteins and fats are broken down into compounds that feed into the glycolysis or citric acid cycle.
- 🏋️♂️ Mitochondria are considered the 'engine of the cell' as they produce most of the energy needed for cellular activities.
Q & A
What are the first two steps of cellular respiration mentioned in the script?
-The first two steps of cellular respiration mentioned are glycolysis and the citric acid cycle.
Why do glycolysis and the citric acid cycle not generate much energy?
-Glycolysis and the citric acid cycle do not generate much energy because they do not produce a large amount of ATP, but rather prepare molecules like NADH and FADH2 for oxidative phosphorylation, which is where most ATP is generated.
What is the role of the electron transport chain in oxidative phosphorylation?
-The electron transport chain in oxidative phosphorylation facilitates a series of redox reactions, transferring electrons through a series of protein complexes (I-IV) and ultimately generating a proton gradient across the mitochondrial membrane.
What are the protein complexes that make up the electron transport chain?
-The protein complexes that make up the electron transport chain are referred to as complexes I-IV.
What is the function of ubiquinone in the electron transport chain?
-Ubiquinone, also known as coenzyme Q or CoQ, is a small hydrophobic molecule that is mobile within the mitochondrial membrane and plays a role in shuttling electrons through the electron transport chain.
How does the proton gradient generated by the electron transport chain lead to ATP synthesis?
-The proton gradient generated by the electron transport chain drives protons back into the mitochondrial matrix through ATP synthase, a process known as chemiosmosis. This movement of protons powers ATP synthase to phosphorylate ADP into ATP.
What is chemiosmosis and how is it related to ATP production?
-Chemiosmosis is the process by which the proton gradient across the mitochondrial membrane is used to drive the synthesis of ATP. Protons flow down their concentration gradient through ATP synthase, which catalyzes the phosphorylation of ADP to ATP.
How many ATP molecules can be generated from a single molecule of glucose through oxidative phosphorylation?
-From a single molecule of glucose, approximately 26 or 28 ATP molecules can be generated through oxidative phosphorylation.
How do proteins, fats, and other carbohydrates contribute to cellular respiration?
-Proteins, fats, and other carbohydrates are initially broken down in unique ways, but eventually, they result in compounds that are fed into the pathways of glycolysis, the citric acid cycle, or oxidative phosphorylation, contributing to ATP production.
Why are mitochondria referred to as the 'engine of the cell'?
-Mitochondria are referred to as the 'engine of the cell' because they are responsible for generating most of the cell's energy through the process of cellular respiration, particularly through oxidative phosphorylation.
What is the significance of the ATP produced by cellular respiration?
-The ATP produced by cellular respiration is significant because it is the primary energy currency of the cell, used to power various cellular processes and activities.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
5.0 / 5 (0 votes)