Methods of Dating the Earth Part 2: Absolute Dating (Radiometric Dating)
Summary
TLDRThis tutorial delves into the evolution of dating methods used by geologists to determine Earth's age. Initially, relative dating provided wide-ranging estimates, from millions to billions of years. The advent of radiometric dating, leveraging nuclear decay, revolutionized the field by offering precise age determinations. Key criteria for successful radiometric dating include rocks forming closed systems post-formation. The script explains the process using potassium-argon and uranium-lead dating, highlighting the importance of isotopes and their decay constants for calculating half-lives. It also introduces isochron dating, which doesn't require the assumption of no initial daughter isotopes. The narrative underscores the reliability of these methods and their profound impact on our understanding of Earth's history.
Takeaways
- 🕰️ Prior to the 1900s, geologists used relative dating to estimate the Earth's age, resulting in varied estimates from millions to billions of years.
- 🔍 The uncertainty in relative dating was due to the difficulty in determining the time represented by missing time unconformities.
- 📈 The advent of radiometric dating provided a more accurate and detailed timeline of Earth's history when combined with relative dating methods.
- 🌋 Radiometric dating, or absolute dating, is based on the nuclear decay of radioactive nuclides to determine the exact age of rocks.
- 💠 For radiometric dating to be effective, rocks must form closed systems with no exchange of atoms with the environment after formation.
- 🌋 The best candidates for radiometric dating are igneous rocks that have cooled quickly and not been reheated above the blocking temperature.
- 🚫 Sedimentary rocks cannot be directly dated using absolute dating, but their constituent mineral grains, like zircon crystals, can be.
- ⛰️ Metamorphic rocks are challenging for radiometric dating due to the potential for hot fluids to alter parent and daughter isotopes during metamorphism.
- 📚 Radiometric dating was first documented in 1907 by Dr. Bertram Boltwood, who discovered the decay of uranium into lead.
- 🔬 The process of radiometric dating involves selecting a suitable rock and isotope system, ensuring the rock's minerals have not been altered, and analyzing them with a mass spectrometer.
- 📈 Isochron dating is a method that does not require the assumption of no initial daughter isotopes, making it useful for certain types of rocks.
Q & A
What was the main issue with relative dating before the advent of radiometric dating?
-The main issue with relative dating was the dramatic variation in estimates of Earth's age, ranging from millions to billions of years, due to the inaccuracy in determining the time represented by missing time unconformities.
How does radiometric dating, or absolute dating, provide more accurate age estimates compared to relative dating?
-Radiometric dating provides exact ages from rocks by utilizing the concept of nuclear decay, where radioactive nuclides emit a high-energy particle to become a nuclide of another element. This method allows for a detailed and accurate timeline of Earth's history.
What are the prerequisite criteria for rocks to be suitable for radiometric dating?
-For radiometric dating, rocks must form and then become closed systems with no exchange of atoms between the rock and its environment. The best candidates are igneous rocks that cooled quickly and have not been reheated above the blocking temperature.
Why are sedimentary rocks not suitable for direct radiometric dating, and what alternative is used instead?
-Sedimentary rocks are not suitable for direct radiometric dating because they cannot be dated using absolute dating methods. However, their constituent mineral grains, such as zircon crystals, can be dated.
How does metamorphism affect the reliability of radiometric dating of metamorphic rocks?
-Metamorphism can involve hot fluids that can add or remove parent and daughter isotopes, making radiometric dating of metamorphic rocks difficult and less reliable.
Who first documented radiometric dating, and what was the key discovery that enabled this method?
-Dr. Bertram Boltwood first documented radiometric dating in 1907 after discovering that uranium decays into lead, and that the uranium to lead ratio in a rock varies based on the rock's age.
What is the significance of isotopes in the context of radiometric dating?
-Isotopes, or atoms of the same element with differing numbers of neutrons, are crucial in radiometric dating because they decay at a specific rate, represented by the isotope's decay constant, which can be used to calculate its half-life.
Why is the half-life of a radioactive isotope considered extremely reliable in radiometric dating?
-The half-life of a radioactive isotope is extremely reliable because it does not depend on any aspect of the environment and remains constant everywhere in the universe under any conditions.
What is the importance of isotopic ratios in determining the age of a geologic material?
-Isotopic ratios are important because they allow comparison with naturally occurring ratios in matter that is being formed and freely exchanged with its environment, enabling the determination of how many half-lives have elapsed and thus the age of the object.
How does isochron dating differ from other forms of radiometric dating?
-Isochron dating does not require the assumption that the material being analyzed did not contain any isotopes of the daughter element at the time it was crystallized, unlike other forms of radiometric dating.
Can you explain the process of determining the age of a rock using the potassium-argon dating system as described in the script?
-To determine the age of a rock using the potassium-argon system, geologists analyze the amount of radiogenic argon in the rock using a mass spectrometer. They use a formula involving the current amount of radiogenic argon, the decay constant, and the fraction of potassium-40 that decays via electron capture to calculate the rock's age.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة

RELATIVE AND ABSOLUTE DATING OF ROCKS / EARTH AND LIFE SCIENCE / SCIENCE 11 - MELC 12 & 13

Relative and Absolute Dating | Earth and Life Science
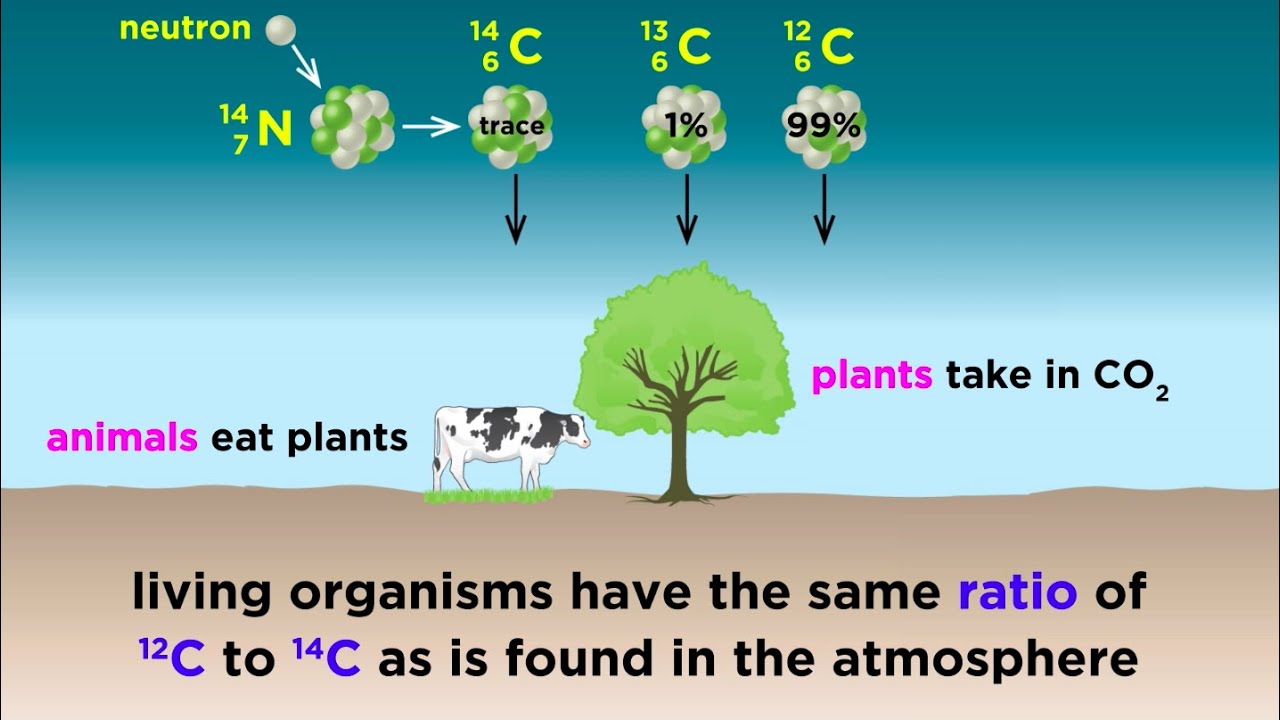
Radiometric Dating: Carbon-14 and Uranium-238

How We Know The Earth Is Ancient

Methods of Dating the Earth Part 1: Relative Dating

Numerical Time (Candy & Radioactive decay)
5.0 / 5 (0 votes)
