Class 12th – Electron theory of Electrification | Electric Charges and Fields | Tutorials Point
Summary
TLDRThis educational transcript delves into electron theories of electrification, explaining the atomic structure with a nucleus carrying a positive charge balanced by electrons' negative charge, resulting in a net charge of zero. It highlights the fundamental charge, or the charge of a single electron, as a key concept, measured in Coulombs. The script explores the exchange of electrons during processes like rubbing, which leads to objects becoming positively or negatively charged. It emphasizes that only electrons, due to their light mass, are responsible for electrification, not protons. The summary concludes with the impact of charging on an object's mass, noting that a negatively charged object gains mass from additional electrons, while a positively charged object loses mass as electrons depart.
Takeaways
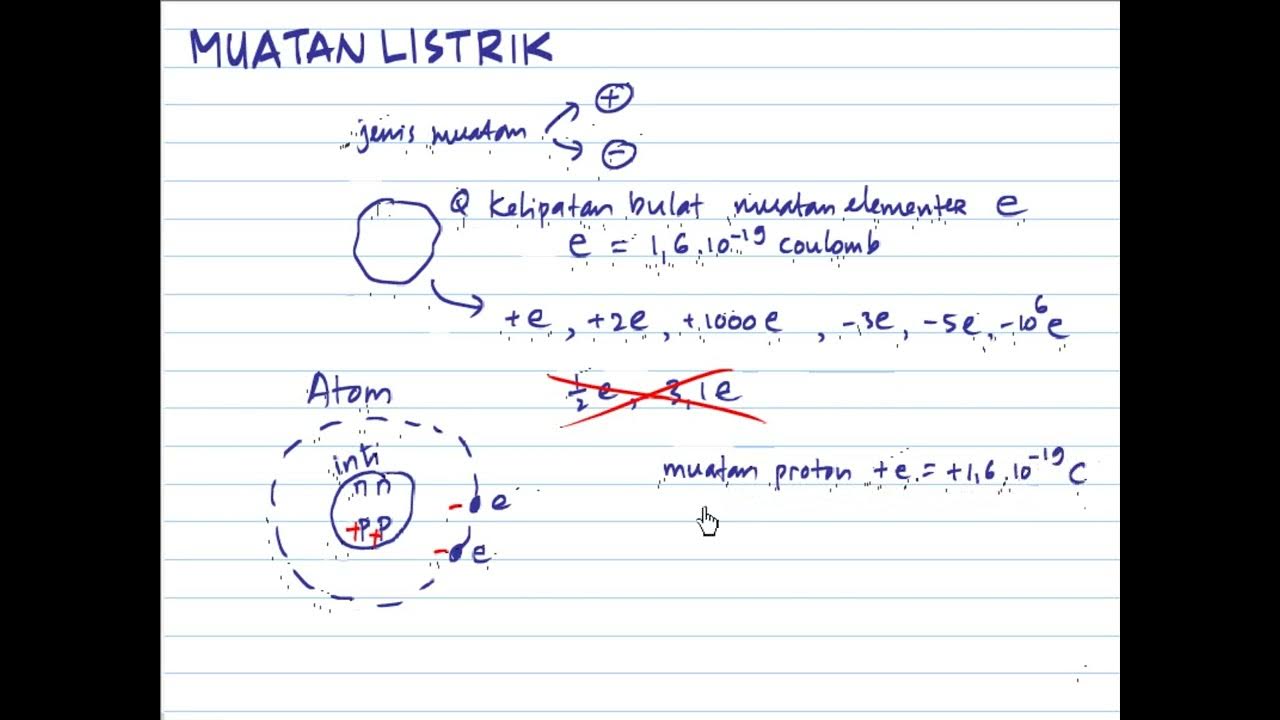
- 🔬 Atoms consist of a positively charged nucleus and negatively charged electrons orbiting around it, resulting in a net charge of zero.
- 🚀 The fundamental charge, or the charge of a single electron, is a critical concept, denoted as 'e' and measured at 1.6 x 10^-19 Coulombs.
- ⚖️ The mass of an atom changes when it becomes charged; gaining electrons (negative charge) increases mass, while losing electrons (positive charge) decreases it.
- 🌐 The universe's net charge is zero due to the balance of equal numbers of protons and electrons in all atoms.
- 🔋 The process of electrification involves the transfer of electrons between objects, leading to one object gaining electrons (negative charge) and the other losing them (positive charge).
- ❌ Protons, being much heavier than electrons, do not typically move between atoms, making electrons solely responsible for electrification.
- 💡 The concept of charging by material transfer is essential; a body becomes negatively charged by gaining electrons and positively charged by losing them.
- 🔑 The electron's lightweight nature makes it the preferred particle for nature to move, influencing the charge state of objects.
- 📚 Understanding electron theory is crucial for grasping the basics of electricity and the behavior of different materials in electrical contexts.
- 📈 The script also hints at upcoming topics, such as the classification of materials into conductors, insulators, and semiconductors, and their electrical properties.
Q & A
What is the net charge of an atom?
-The net charge of an atom is zero because it consists of an equal number of electrons with negative charges and protons with positive charges in the nucleus.
What is the fundamental charge?
-The fundamental charge is the charge on one electron, which is the smallest unit of electric charge. It is 1.6 x 10^-19 Coulombs and is represented by the symbol 'e'.
Why are protons not exchanged during electrification processes?
-Protons are not exchanged during electrification processes because they are located in the nucleus of the atom and are much heavier than electrons. The mass of a proton is 1837 times that of an electron, making it less mobile and less likely to be transferred between atoms.
How does the mass of an object change when it becomes charged?
-When an object becomes negatively charged, its mass increases because electrons (which have mass) are added to it. Conversely, when an object becomes positively charged, its mass decreases as electrons are removed from it.
What is the role of electrons in the process of electrification?
-Electrons are solely responsible for electrification. They can be transferred between atoms, leading to an excess (negative charge) or deficiency (positive charge) of electrons in a material.
What happens during the process of rubbing two objects together?
-When two objects are rubbed together, there is a transfer of electrons from one object to another, resulting in one object becoming negatively charged (excess electrons) and the other becoming positively charged (deficiency of electrons).
Why are electrons considered lightweight in comparison to protons?
-Electrons are considered lightweight because their mass is significantly smaller than that of protons. This makes it easier for electrons to move between atoms, which is why they are involved in the process of electrification.
What is the significance of the number 1.6 x 10^-19 Coulombs in the context of the script?
-The number 1.6 x 10^-19 Coulombs represents the charge of a single electron, known as the fundamental charge. It is a standard measure used in physics to describe the magnitude of electric charge on subatomic particles.
How does the presence of an electron affect the charge of an atom?
-The presence of an electron contributes to the negative charge of an atom. If an atom gains an extra electron, it becomes negatively charged, and if it loses an electron, it becomes positively charged.
What are the two types of charges that can result from the transfer of electrons between atoms?
-The two types of charges that can result from the transfer of electrons between atoms are positive charge, which occurs when an atom loses electrons, and negative charge, which occurs when an atom gains excess electrons.
What is the relationship between the mass of an electron and the change in mass of a body when it becomes charged?
-The mass of an electron is 9.1 x 10^-31 kg. When a body becomes charged, its mass changes due to the addition or removal of electrons. If a body gains electrons, its mass increases, and if it loses electrons, its mass decreases.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآن5.0 / 5 (0 votes)