Termoquímica - Brasil Escola
Summary
TLDRIn this video, Professor Choven breaks down the key concepts of thermochemistry, focusing on the enthalpy, the heat involved in chemical reactions. He explains how to calculate the variation in enthalpy (ΔH) by comparing the heat of the reactants and products. The video covers exothermic and endothermic reactions, providing clear explanations and visual aids. Additionally, it explores various types of reaction heat, including formation, combustion, and bond dissociation heats, along with the concept of Hess's Law. Practical tips and examples are given to help viewers better understand and identify these concepts in real-world reactions.
Takeaways
- 😀 Thermochemistry is the branch of chemistry that studies the heat involved in chemical reactions.
- 😀 Enthalpy (ΔH) represents the heat content of a system and is crucial in thermochemistry.
- 😀 The change in enthalpy (ΔH) can be calculated as ΔH = H(products) - H(reactants).
- 😀 Factors affecting ΔH include the physical state of reactants and products, their allotropes, temperature, and concentration.
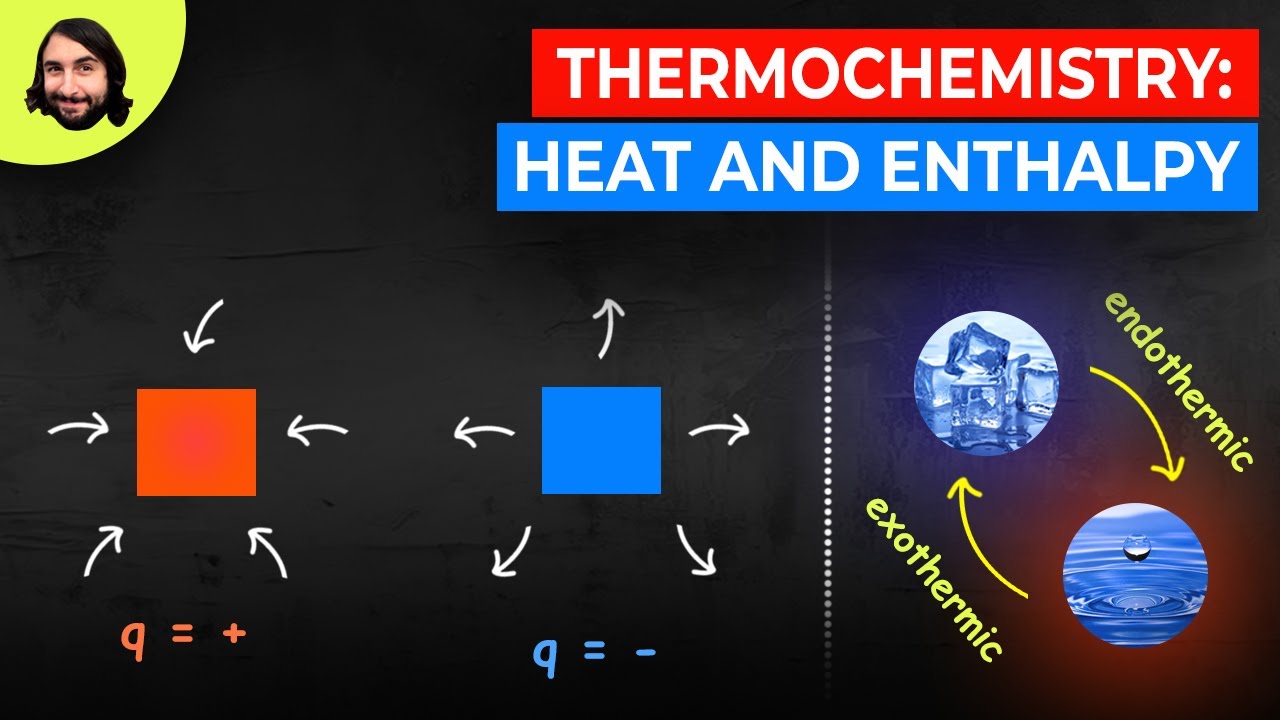
- 😀 Exothermic reactions release heat and have a negative ΔH, while endothermic reactions absorb heat and have a positive ΔH.
- 😀 A visual representation of exothermic reactions shows a decrease in energy after the reaction, resulting in negative ΔH.
- 😀 In endothermic reactions, energy is absorbed, resulting in an increase in energy and a positive ΔH.
- 😀 The heat of formation refers to the energy released or absorbed when one mole of a substance is formed from its elements in their standard states.
- 😀 The heat of combustion is the energy released during the combustion of one mole of a substance at standard conditions (25°C, 1 ATM).
- 😀 The heat of bond dissociation is the energy needed to break one mole of bonds in a gaseous substance at standard conditions.
- 😀 Hess's Law states that the total heat change of a reaction is the same, regardless of the path taken, and that reversing or multiplying reactions will affect the ΔH accordingly.
Q & A
What is thermochemistry?
-Thermochemistry is the branch of chemistry that studies the heat involved in chemical processes, including the heat released or absorbed during chemical reactions.
What is enthalpy, and how is it related to thermochemistry?
-Enthalpy is the quantity of heat contained within a system. In thermochemistry, it is used to understand the energy changes that occur before and after a chemical reaction, helping to calculate the heat released or absorbed during a reaction.
How do you calculate the change in enthalpy (ΔH) during a chemical reaction?
-The change in enthalpy (ΔH) is calculated by subtracting the enthalpy of the reactants from the enthalpy of the products: ΔH = H(products) - H(reactants).
What factors influence the value of ΔH?
-The factors that influence ΔH include the physical state of the reactants and products, their allotropic forms, temperature, and concentration.
What are exothermic reactions, and how can you identify them?
-Exothermic reactions are those that release heat. You can identify them by a negative ΔH value (ΔH < 0), a decrease in energy during the reaction, and when heat is listed among the products in a chemical equation.
What are endothermic reactions, and how are they identified?
-Endothermic reactions absorb heat during the reaction. They are characterized by a positive ΔH value (ΔH > 0), an increase in energy, and when heat is listed among the reactants in a chemical equation.
What is the heat of formation in thermochemistry?
-The heat of formation is the energy released or absorbed when one mole of a substance is formed from its elements in their standard state (at 1 ATM pressure and 25°C).
What is the heat of combustion?
-The heat of combustion is the energy released when one mole of a substance undergoes combustion (burning) in oxygen at 25°C and 1 ATM pressure. It is typically an exothermic reaction.
What is the heat of bond dissociation, and what conditions are required for it?
-The heat of bond dissociation refers to the energy required to break one mole of bonds in a molecule in its gaseous state, at 1 ATM pressure and 25°C.
What does Hess's Law state, and how does it apply to thermochemical calculations?
-Hess's Law states that the total heat change in a reaction is the same, regardless of the reaction pathway. This means that energy changes can be calculated by summing the heat changes of individual steps, provided the initial and final states are the same.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)