Proceso isobárico - Isobaric process
Summary
TLDRThis video explains the concept of an isobaric process in thermodynamics, where pressure remains constant while volume changes. The term 'bar' refers to pressure, and the work done during an isobaric process is calculated by multiplying pressure by the change in volume. Using a balloon as an example, the video demonstrates how supplying heat to the system causes the air inside to expand while keeping the pressure constant. The ideal gas law is applied to relate temperature, volume, and pressure changes. This process is crucial in understanding energy exchanges in thermodynamic systems.
Takeaways
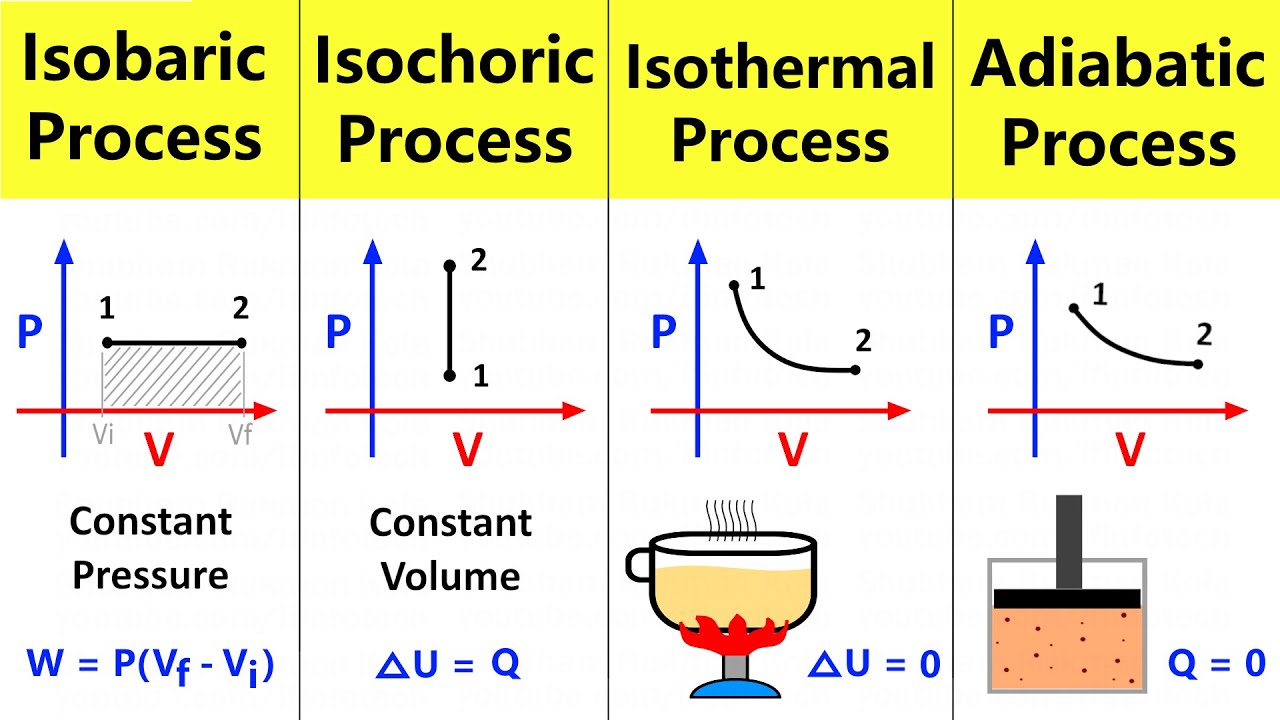
- 😀 An isobaric process is a thermodynamic process where the pressure remains constant while the volume changes.
- 😀 The word 'isobaric' comes from the Greek word 'baros,' meaning pressure.
- 😀 Pressure is a key concept in an isobaric process, and the work done is calculated using the formula: W = P * ΔV.
- 😀 In an isobaric process, the volume of the gas can change, but the pressure stays the same.
- 😀 The example of a balloon is used to demonstrate an isobaric process, where heating the gas inside causes the balloon to expand.
- 😀 As the temperature inside the balloon increases, the internal energy of the air increases, causing the gas to expand and the volume to increase.
- 😀 The first law of thermodynamics states that the heat supplied to a system is equal to the change in internal energy plus the work done by the system.
- 😀 The ideal gas law, P = nRT / V, can be used in an isobaric process to describe the relationship between pressure, temperature, and volume.
- 😀 In an isobaric process, the ratio of temperature to volume remains constant, and we can use the equation: (T1 / V1) = (T2 / V2).
- 😀 The work done in an isobaric process is the product of the constant pressure and the change in volume, measured in units of joules or pascals.
- 😀 Understanding isobaric processes is crucial in studying the behavior of gases under different conditions, especially in thermodynamics.
Q & A
What does an isobaric process mean in thermodynamics?
-An isobaric process is a thermodynamic process in which the pressure remains constant while the volume changes. The term 'isobaric' comes from the Greek word 'baros,' meaning pressure.
What is the significance of the term 'bar' in an isobaric process?
-'Bar' is a unit of pressure derived from the Greek word 'baros,' which means weight or pressure. It is commonly used to measure pressure in thermodynamics.
How is work calculated in an isobaric process?
-The work (W) done in an isobaric process is calculated as the product of pressure (P) and the change in volume (ΔV). The formula is W = P * ΔV, where ΔV is the difference between the final and initial volumes (V2 - V1).
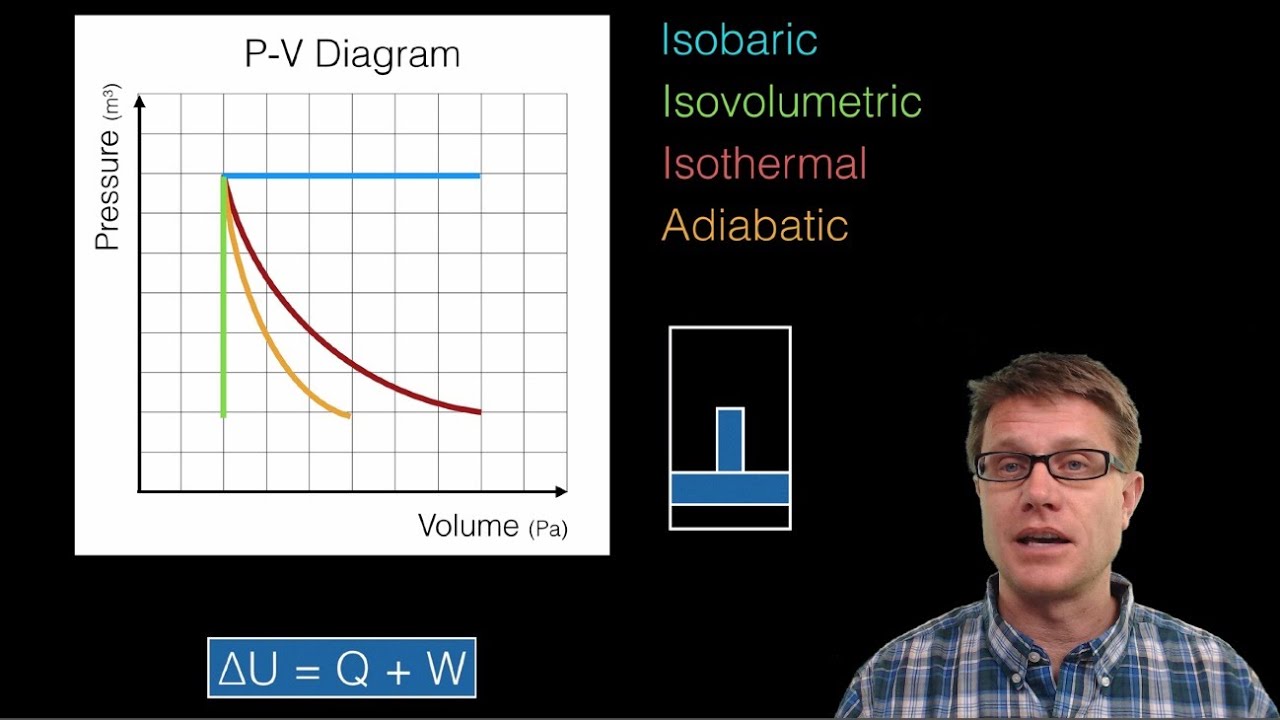
What does the area under the pressure-volume curve represent in an isobaric process?
-The area under the pressure-volume (P-V) curve in an isobaric process represents the work done by the system. Since pressure is constant, the area forms a rectangle, and the work is the product of pressure and the change in volume.
What is the first law of thermodynamics, and how does it apply to isobaric processes?
-The first law of thermodynamics states that the heat supplied to a system (Q) equals the change in internal energy (ΔU) plus the work done by the system (W). In an isobaric process, heat causes an increase in the internal energy of the gas and also does work on the surroundings.
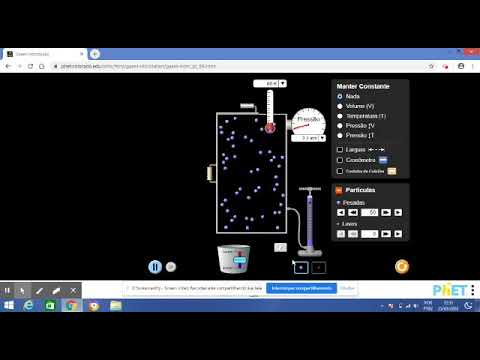
How does heating a balloon demonstrate an isobaric process?
-When heat is applied to a balloon, the internal energy of the air inside increases, causing the air molecules to vibrate more and expand. Since the pressure inside the balloon remains equal to the external pressure, this is an isobaric process. The balloon's volume increases as the temperature rises.
How does the ideal gas law relate to an isobaric process?
-The ideal gas law (P = nRT/V) relates pressure, temperature, volume, and the number of moles of gas. In an isobaric process, where pressure remains constant, the relationship between temperature and volume is governed by the equation T1/V1 = T2/V2, allowing for calculations of changes in temperature and volume.
What is the significance of the temperature-volume relationship in an isobaric process?
-In an isobaric process, the temperature and volume of a gas are directly proportional when pressure is constant. This relationship is expressed by the equation T1/V1 = T2/V2, meaning that as the temperature increases, the volume also increases, and vice versa.
How do you calculate the work done in an isobaric process when given the pressure and volume changes?
-The work done in an isobaric process can be calculated using the formula W = P * (V2 - V1), where P is the constant pressure and V2 and V1 are the final and initial volumes, respectively.
What role does the constant gas constant (R) play in an isobaric process involving an ideal gas?
-In an isobaric process involving an ideal gas, the gas constant (R) is a key component in the ideal gas law (P = nRT/V). While R remains constant, the relationship between pressure, temperature, and volume can be used to analyze the changes in these parameters during the process.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)