COMO FUNCIONAM AS PILHAS E AS BATERIAS (ELETROQUÍMICA) | Resumo de Química Enem. Prof Felipe Sobis
Summary
TLDRThis video lesson introduces the concept of electrochemistry, focusing on galvanic cells (pilhas). It explains how a spontaneous chemical reaction between two metals, such as zinc and copper, generates electrical energy. The process of oxidation (loss of electrons) and reduction (gain of electrons) is covered, with zinc acting as the anode (negative) and copper as the cathode (positive). The flow of electrons through the circuit produces a current that can power devices. Key terms like salt bridge and electrode are also introduced to explain how charge balance is maintained, making the concept accessible and engaging for students.
Takeaways
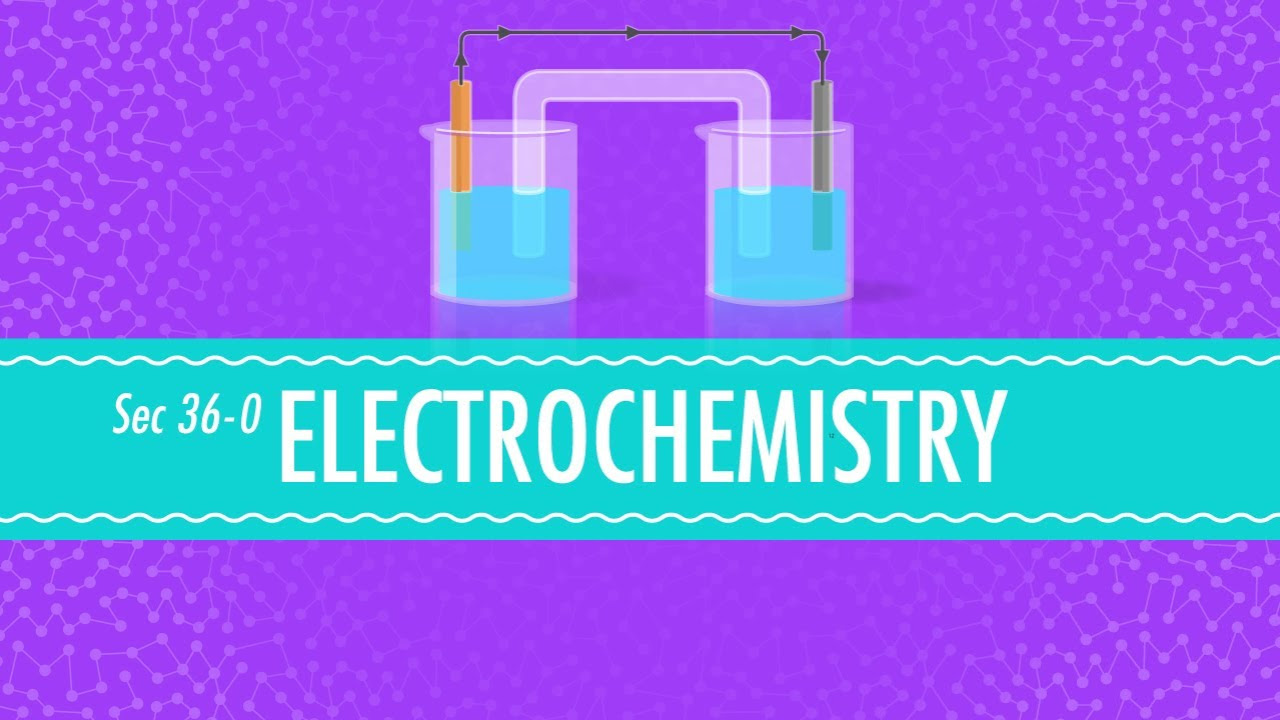
- 😀 A galvanic cell (like a Daniel's cell) generates electricity through spontaneous chemical reactions.
- 😀 Oxidation occurs when a substance loses electrons and becomes more positive (e.g., zinc losing electrons).
- 😀 Reduction happens when a substance gains electrons and becomes more negative (e.g., copper ions gaining electrons).
- 😀 In a galvanic cell, zinc undergoes oxidation at the anode (negative electrode), and copper undergoes reduction at the cathode (positive electrode).
- 😀 The flow of electrons from the zinc anode to the copper cathode generates electrical current.
- 😀 The salt bridge is crucial for maintaining electrical neutrality and allowing the reactions to continue by providing ion flow between the two solutions.
- 😀 Zinc dissolves into the solution as Zn²⁺, causing the zinc electrode to lose mass during oxidation.
- 😀 Copper ions (Cu²⁺) gain electrons and deposit as solid copper, increasing the mass of the copper electrode during reduction.
- 😀 The galvanic cell powers devices like lamps through the movement of electrons, which is a practical example of electrochemical principles.
- 😀 Mobile phones use a galvanic cell while discharging (powering the phone) and electrolysis while charging (reversing the discharge process).
- 😀 The key takeaway for identifying the electrodes: oxidation occurs at the anode (negative) and reduction at the cathode (positive).
Q & A
What is the main focus of the video?
-The video focuses on explaining the basics of electrochemistry, particularly how a galvanic cell (specifically the Daniel cell) works to generate electrical energy from chemical reactions.
What is a galvanic cell?
-A galvanic cell is an electrochemical device where a spontaneous chemical reaction occurs, generating electrical energy. In the case of the Daniel cell, it consists of two metal electrodes and their corresponding salts.
What is the difference between oxidation and reduction?
-Oxidation is the process where a substance loses electrons and becomes more positive, while reduction is the process where a substance gains electrons and becomes more negative.
What happens at the anode of a galvanic cell?
-At the anode, oxidation occurs. In the Daniel cell, the zinc electrode loses electrons and dissolves into the solution as zinc ions (Zn²⁺). The anode is considered the negative terminal.
What happens at the cathode of a galvanic cell?
-At the cathode, reduction occurs. In the Daniel cell, copper ions (Cu²⁺) in the solution gain electrons and are deposited as copper metal on the electrode. The cathode is considered the positive terminal.
How does the salt bridge function in a galvanic cell?
-The salt bridge connects the two half-cells and allows ions to flow between them, maintaining electrical neutrality. This prevents the buildup of charge that would stop the flow of electrons.
What role do the zinc and copper electrodes play in the Daniel cell?
-In the Daniel cell, the zinc electrode acts as the anode where oxidation occurs (zinc loses electrons), and the copper electrode acts as the cathode where reduction occurs (copper ions gain electrons and are deposited as copper metal).
Why does the zinc electrode lose mass in the Daniel cell?
-The zinc electrode loses mass because zinc atoms undergo oxidation, releasing zinc ions (Zn²⁺) into the solution. As the zinc atoms lose electrons, the electrode gradually dissolves.
What is the significance of the electron flow in the circuit?
-The flow of electrons from the anode (zinc electrode) to the cathode (copper electrode) through an external circuit generates an electric current, which can be used to power devices, such as a light bulb.
How can we determine the polarity of the electrodes in a galvanic cell?
-The anode is the negative terminal because it undergoes oxidation (loses electrons), and the cathode is the positive terminal because it undergoes reduction (gains electrons). This is often remembered by the mnemonic 'An Ox, Red Cat' (Anode - Oxidation, Reduction - Cathode).
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)