Hakikat Ilmu Kimia Kelas 10 • Part 1: Hakikat dan Peran Ilmu Kimia dalam Kehidupan
Summary
TLDRThis video from 'Jendela Sains' explores the fundamental aspects of chemistry, its role in daily life, and its branches. It delves into the nature of chemistry, studying the composition, structure, properties, and changes of matter. The script highlights the significance of chemistry in various fields such as medicine, agriculture, geology, biology, law, and engineering, and discusses its role in addressing global issues like alternative energy sources, biogas technology, and reducing air pollution with catalytic converters.
Takeaways
- 🧪 Chemistry is the science that studies the reactions between elements or compounds and their molecular combinations to form new compounds.
- 🔍 There are four fundamental aspects of chemistry: the study of the composition, structure, properties, and changes of matter.
- 🌐 The composition of matter reveals the types of atoms or elements that make up a substance and how they are bonded together, such as H2O being made of hydrogen and oxygen.
- 📐 The structure of matter provides a picture of how atoms are bonded, like the V-shaped structure of a water molecule.
- 🔬 Properties of matter encompass both physical properties (appearance and state) and chemical properties (reactivity and bonding with other substances).
- 🔄 The change in matter includes physical changes that can be reversed and chemical changes that result in new substances and are irreversible, like the burning of paper.
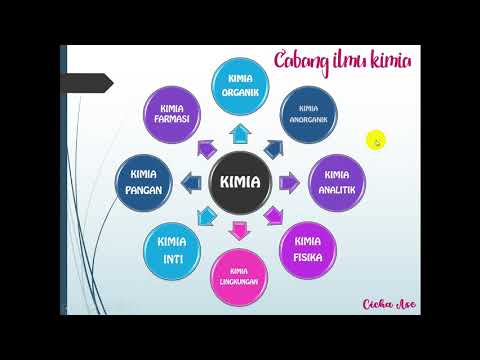
- 🌿 Organic chemistry focuses on the structure, composition, reactions, and synthesis of organic compounds.
- 🏗️ Analytical chemistry investigates the qualitative and quantitative aspects of substances within a sample, such as identifying and measuring the amount of a substance.
- 🌳 Biochemistry explores the properties and composition of substances within living organisms and their transformations.
- ♻️ Environmental chemistry studies environmental issues like pollution and waste management, dealing with chemical substances that cause contamination.
- 💊 Pharmaceutical chemistry is concerned with the isolation of active natural substances, and the development of beneficial medicinal compounds.
- 🔥 The benefits of chemistry are vast, including its role in medicine for drug interactions and discoveries, agriculture for soil fertility and fertilizer formulation, geology for understanding minerals, biology for studying life processes, and forensics for legal examinations.
Q & A
What is the main subject of the 'Jendela Sains' video?
-The main subject of the video is the essence and role of chemistry in life.
What are the four fundamental aspects of chemistry according to the video?
-The four fundamental aspects are the study of the composition of matter, the structure of matter, the properties of matter, and the changes in matter.
What does the composition of matter in chemistry involve?
-It involves the types of atoms or elements that make up a substance and how these atoms or elements are bonded together.
Can you explain the structure of matter as discussed in the video?
-The structure of matter refers to the arrangement of atoms that are bonded together, such as the molecular structure of water (H2O).
What are the physical properties of matter that the video mentions?
-Physical properties include the state of matter (solid, liquid, or gas), appearance, color, and smell, which can be perceived by the senses.
How does the video differentiate between chemical and physical changes?
-Chemical changes result in the formation of new substances and cannot be reversed, while physical changes do not produce new substances and can be reversed, such as the evaporation of water.
What are the nine branches of chemistry briefly mentioned in the video?
-The branches include organic chemistry, inorganic chemistry, analytical chemistry, biochemistry, environmental chemistry, pharmaceutical chemistry, physical chemistry, food chemistry, and nuclear chemistry.
How does chemistry play a role in the medical field as per the video?
-Chemistry helps in understanding how drugs interact with diseases, supporting the discovery of new medicines.
What is the role of chemistry in agriculture according to the video?
-Chemistry provides information about the chemical composition of soil and its fertility, supporting the formulation of synthetic fertilizers.
How does chemistry contribute to environmental issues as discussed in the video?
-Chemistry studies environmental issues like pollution and waste management, focusing on the chemical substances that cause contamination.
What are the three global issues that the video highlights where chemistry plays a significant role?
-The issues are the discovery of alternative energy sources like bioethanol, the use of biogas technology utilizing methane from livestock waste, and the 'Blue Sky' program aimed at minimizing air pollution through catalytic converters.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)