A Level Chemistry Revision "Polar Bonds and Polar Molecules".
Summary
TLDRIn this video, the concept of polar and non-polar molecules is explored. It begins by explaining electronegativity and how it affects bond polarity. The video uses examples such as chlorine (Cl2) and hydrogen chloride (HCl) to show pure covalent and polar covalent bonds. The discussion then expands to molecules like carbon dioxide (CO2) and tetrachloromethane, demonstrating how molecular symmetry can lead to non-polar molecules despite having polar bonds. Water is highlighted as a classic example of a polar molecule due to its bent structure, which prevents dipoles from canceling out. The video sets the stage for exploring intermolecular forces in the next episode.
Takeaways
- 😀 Different elements have different electronegativities, which is their ability to attract electrons in a covalent bond.
- 😀 The most electronegative elements are located at the top right of the periodic table.
- 😀 A pure covalent bond occurs when both atoms in a molecule have the same electronegativity, as seen in the chlorine molecule (Cl2).
- 😀 A polar covalent bond occurs when one atom is more electronegative than the other, causing the electron pair to shift toward the more electronegative atom, as seen in hydrogen chloride (HCl).
- 😀 The separation of charge in a polar bond creates a dipole, represented by a delta-positive (δ+) and delta-negative (δ-) symbol.
- 😀 An alternative way to show bond polarity is by using an arrow that points towards the more electronegative element.
- 😀 The dipole moment refers to the overall polarity of a molecule that has a polar covalent bond.
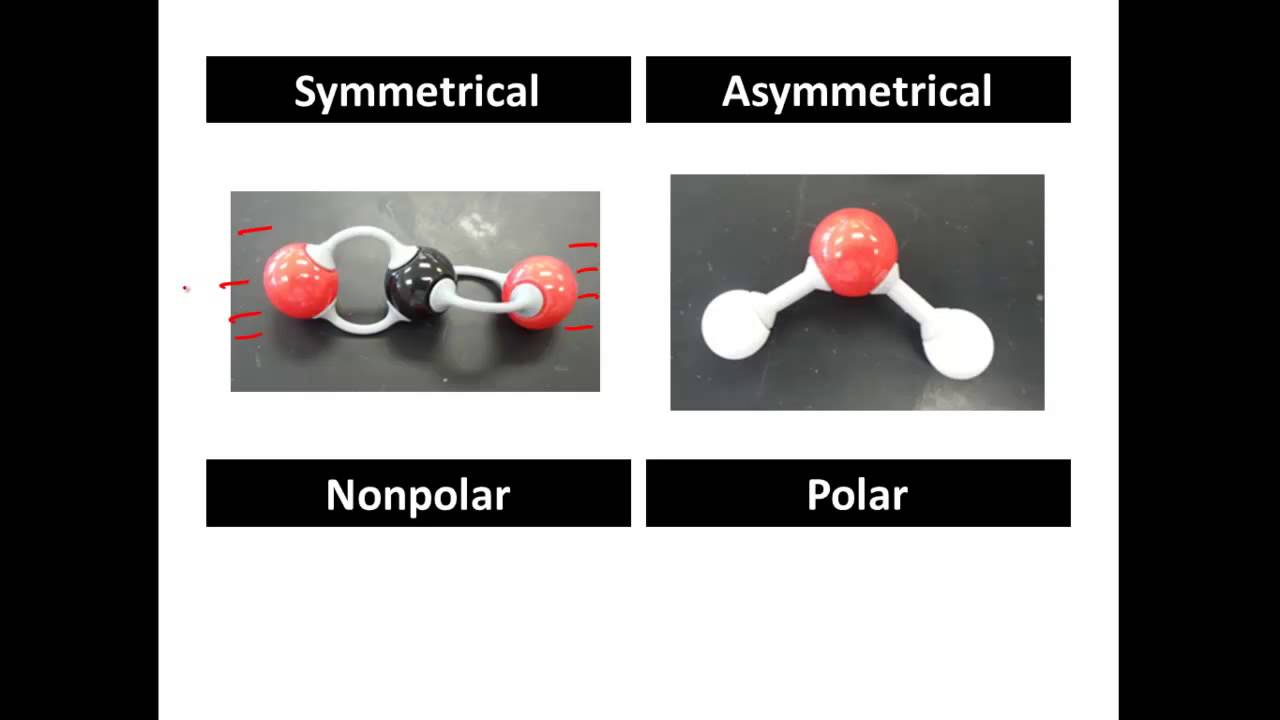
- 😀 In molecules like carbon dioxide (CO2) with two polar bonds, the dipoles cancel out if the molecule is linear, resulting in no overall polarity.
- 😀 Molecules with symmetrical structures, like tetrachloromethane (CCl4), also have canceling dipoles, making them non-polar.
- 😀 In molecules like trichloromethane (CHCl3), the dipoles do not cancel out because of an asymmetrical shape, making it a polar molecule.
- 😀 Water (H2O) has a non-linear shape, and the polar bonds do not cancel, making it a polar molecule.
Q & A
What is electronegativity?
-Electronegativity is the ability of an atom to attract the pair of electrons in a covalent bond.
Why is the bond in a chlorine molecule (Cl2) described as a pure covalent bond?
-In Cl2, both atoms are chlorine and have the same electronegativity, so the shared electrons lie midway between them, resulting in no charge separation or dipole.
Why is the bond in hydrogen chloride (HCl) considered polar?
-Chlorine has a higher electronegativity than hydrogen, pulling the shared electrons closer to itself. This creates a separation of charge, known as a dipole, making the bond polar.
What symbol is used to represent partial charges in polar bonds?
-Partial charges are represented using delta (δ) symbols: δ+ for the slightly positive atom and δ− for the slightly negative atom.
What is another way to show the direction of bond polarity besides delta notation?
-Bond polarity can also be shown with an arrow pointing toward the more electronegative element, with a small cross on the end near the less electronegative atom.
What determines whether a molecule as a whole is polar or non-polar?
-A molecule’s overall polarity depends on both the polarity of its bonds and its shape. If the bond dipoles cancel out due to symmetry, the molecule is non-polar; if they don’t, it is polar.
Why is carbon dioxide (CO2) a non-polar molecule even though it has polar bonds?
-CO2 has two polar carbon-oxygen bonds that are arranged in a straight line, pointing in opposite directions. The dipoles cancel each other out, making the molecule non-polar.
Why is tetrachloromethane (CCl4) non-polar despite having polar C–Cl bonds?
-CCl4 is symmetrical in all directions, so the four C–Cl bond dipoles cancel each other out, resulting in no overall polarity.
Why is trichloromethane (CHCl3) a polar molecule?
-In CHCl3, the C–Cl bonds are polar while the C–H bond is nearly non-polar. The dipoles do not cancel because the molecule is not symmetrical, leaving one side slightly positive and the other negative.
Why is water (H2O) a polar molecule?
-Water has two polar O–H bonds and a bent (non-linear) shape. The bond dipoles do not act in a straight line, so they cannot cancel each other out, giving water an overall polarity.
What is meant by a molecule’s dipole moment?
-The dipole moment is the overall polarity of a molecule, resulting from the vector sum of all its individual bond dipoles.
What happens to the polarity when bonds are arranged symmetrically in a molecule?
-When polar bonds are arranged symmetrically, their dipoles cancel each other out, leading to a non-polar molecule despite the presence of polar bonds.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
Polar and Non Polar Covalent Molecules, Polar vs. Nonpolar - CLEAR & SIMPLE

Polar and NonPolar Molecules: How To Tell If a Molecule is Polar or Nonpolar

Dipole Induced Dipole IMF

POLARITY OF MOLECULES - Part I | ELECTRONEGATIVITY DIFFERENCE | Physical Science

Sifat Kepolaran Senyawa Kovalen

Ikatan Kimia (8) | Cara Menentukan Molekul Polar dan Non Polar | Kimia Kelas 10
5.0 / 5 (0 votes)