Curso de Físico-Química - Gases Parte 5: Comportamento dos Gases Reais e Desvios da Idealidade
Summary
TLDRThis video explores the limitations of the ideal gas law in predicting the behavior of real gases, focusing on phase transitions and deviations from ideality. The speaker discusses Boyle's, Charles’s, and Van der Waals laws, illustrating how real gases, such as helium, neon, and argon, behave differently from ideal gases under varying pressure. The concept of the compressibility factor (Z) is introduced, along with the challenges in predicting phase changes like solid to liquid or gas transitions. The video emphasizes the need for corrections in gas equations to account for these real-world phenomena, particularly the forces between gas molecules that the ideal gas law overlooks.
Takeaways
- 😀 The ideal gas law (PV = nRT) describes the relationship between pressure, volume, temperature, and the number of moles for an ideal gas.
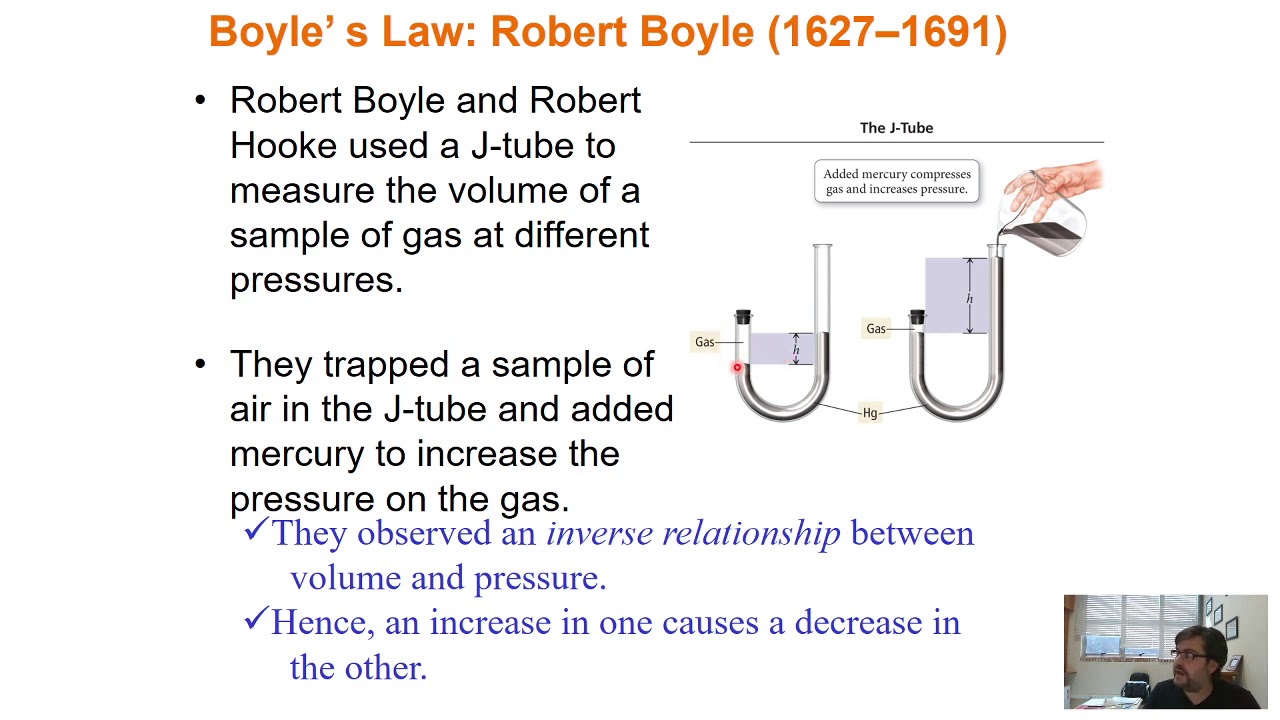
- 😀 Boyle's Law states that the volume of a gas is inversely proportional to its pressure, provided the temperature is constant.
- 😀 Charles's Law states that the volume of a gas is directly proportional to its temperature, provided the pressure is constant.
- 😀 The behavior of gases deviates from ideality when high pressures and certain gases, like helium and neon, are involved.
- 😀 Real gases do not perfectly follow the ideal gas law; deviations occur due to factors like molecular interactions and volume occupied by molecules.
- 😀 The Van der Waals equation was developed to account for the non-ideal behavior of gases, incorporating corrections for intermolecular forces and molecular volumes.
- 😀 The compressibility factor (Z) helps describe how real gases deviate from ideal gas behavior.
- 😀 The ideal gas law cannot predict phase changes like the transition between solid, liquid, and gas states.
- 😀 Phase transitions (e.g., from gas to liquid) involve changes in molecular interactions that the ideal gas law doesn't account for.
- 😀 Van der Waals and other researchers were motivated to improve the gas equation due to their observations of phase changes and non-ideal gas behavior.
- 😀 The real gas equation and corrections to the ideal gas law are necessary to describe phase transitions and other behaviors in gases more accurately.
Q & A
What is the ideal gas law and how does it relate to Boyle's and Charles's laws?
-The ideal gas law is a mathematical equation, PV = nRT, that describes the relationship between pressure (P), volume (V), temperature (T), and the amount of gas (n). Boyle’s law describes how pressure and volume are inversely proportional at a constant temperature, while Charles’s law shows how volume and temperature are directly proportional at a constant pressure. Both are simplifications of the ideal gas law under specific conditions.
Why do real gases deviate from ideal gas behavior?
-Real gases deviate from ideal gas behavior because ideal gas law assumes no interactions between molecules and that they occupy no volume. However, real gas molecules have intermolecular forces (attractions and repulsions), and their volume is not negligible, leading to deviations from ideal behavior, especially under high pressure or low temperature.
What is the Van der Waals equation and why is it important?
-The Van der Waals equation is a modified version of the ideal gas law that accounts for the finite volume of gas molecules and intermolecular forces. It is important because it provides a more accurate description of real gas behavior, especially under conditions where ideal gas law fails.
How do real gases like helium, neon, and argon behave differently from an ideal gas?
-Real gases such as helium, neon, and argon exhibit deviations from the ideal gas behavior, particularly at high pressures. These gases show varying degrees of compressibility, with helium showing a significant variation in volume at high pressures compared to neon or argon, which indicates differences in their molecular interactions.
What is the compressibility factor (Z) and what does it represent?
-The compressibility factor (Z) is a term that quantifies how much a real gas deviates from ideal behavior. It is defined as the ratio of the molar volume of a real gas to the molar volume predicted by the ideal gas law (PV = nRT). A Z value of 1 indicates ideal gas behavior, while values different from 1 indicate deviations due to intermolecular forces or molecular volume.
Why can't the ideal gas law predict phase changes, such as the transition from solid to liquid or liquid to gas?
-The ideal gas law cannot predict phase changes because it ignores molecular interactions, which are crucial in determining the state of matter. Phase changes involve changes in the distance and arrangement of molecules, which are governed by attractive and repulsive forces that the ideal gas law does not account for.
What role do molecular interactions play in the behavior of real gases?
-Molecular interactions, such as attraction and repulsion between gas molecules, significantly affect the behavior of real gases. These forces influence how gases behave under varying conditions of pressure and temperature, leading to deviations from ideal gas behavior, which is why corrections like the Van der Waals equation are necessary.
How does the behavior of gases change across different elements like noble gases?
-Noble gases, despite being chemically similar, exhibit slight variations in behavior due to differences in atomic size and intermolecular forces. These variations lead to different compressibility factors and different responses to changes in pressure and temperature, necessitating adjustments to the ideal gas law for accurate modeling.
What is the significance of studying real gas behavior in relation to phase transitions?
-Studying real gas behavior is crucial for understanding phase transitions because gases do not always behave ideally, especially near critical points where changes from one phase to another occur. Correcting the ideal gas law using real gas equations like the Van der Waals equation helps explain and predict phenomena such as the condensation of gas into liquid.
What motivated researchers like Boyle, Charles, and Van der Waals to develop modifications to the ideal gas law?
-Researchers like Boyle, Charles, and Van der Waals were motivated to develop modifications to the ideal gas law after observing that real gases did not behave as predicted by the ideal gas law, especially under extreme conditions of pressure and temperature. These modifications, such as the Van der Waals equation, account for molecular volume and intermolecular forces, improving the accuracy of gas behavior predictions.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)