Bioquímica - Aula 02 - Alguns conceitos químicos importantes - 1
Summary
TLDRIn this lesson, Professor Ângelo Cortelazzo delves into the fundamental chemical principles necessary for understanding metabolic pathways, including catabolism and anabolism. The lecture covers key concepts like reaction rates, the importance of molecular collisions, temperature effects, and the impact of concentration on reaction speed. The professor explains chemical reaction mechanisms, the role of acids and bases, and introduces Bronsted-Lowry theory. He also explores the dissociation of acids and bases in aqueous solutions and highlights the differences between strong, moderate, and weak acids. The session concludes with a reminder to review general chemistry concepts for a deeper understanding.
Takeaways
- 😀 Chemical reactions occur when molecules collide with sufficient intensity to break bonds and form new substances.
- 😀 Increasing the concentration of reactants increases the likelihood of molecular collisions, thus speeding up the reaction.
- 😀 Higher temperatures cause molecules to move faster, increasing the probability of collisions with sufficient intensity to drive reactions.
- 😀 The position of molecular collisions is crucial, especially for large molecules, as only certain areas of the molecule may be reactive.
- 😀 Chemical reactions in organic chemistry are generally slower than inorganic reactions due to the size and complexity of organic molecules.
- 😀 The rate of a chemical reaction is proportional to the concentration of reactants raised to an exponent, as described by the rate law.
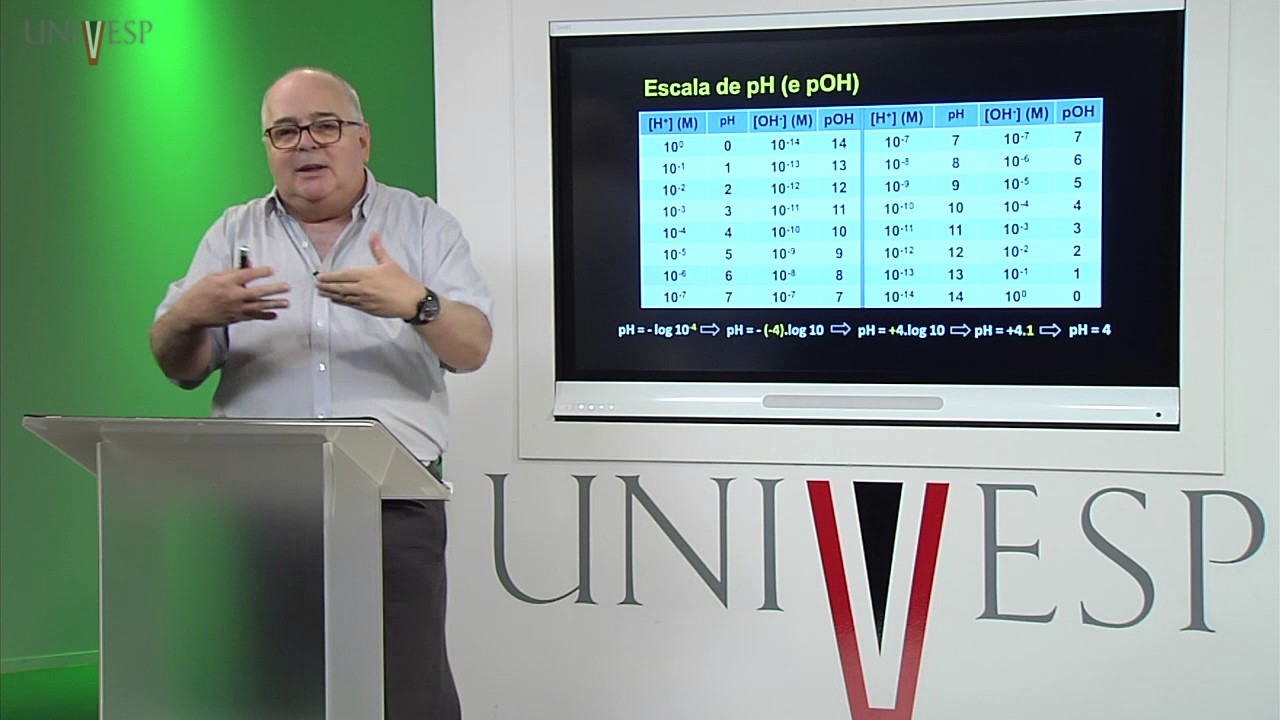
- 😀 Acids are substances that release hydrogen ions (H+) in aqueous solutions, and their strength is determined by how completely they dissociate.
- 😀 A strong acid dissociates completely in water, while a weak acid only partially dissociates, and the dissociation is measured by a coefficient called 'alpha'.
- 😀 A base is a substance that can accept a proton (H+), and its strength is also related to how completely it dissociates in water.
- 😀 Irreversible reactions occur when the products are formed in such a way that they cannot be converted back to the reactants.
- 😀 The Brønsted-Lowry theory expanded the concept of acids and bases by defining acids as proton donors and bases as proton acceptors, forming conjugate acid-base pairs.
Q & A
What is the importance of molecular collision in chemical reactions?
-Molecular collision is crucial because for a chemical reaction to occur, molecules must collide with sufficient energy to break existing bonds and form new ones. Without this collision, the reaction won't take place.
How does temperature affect the rate of a chemical reaction?
-An increase in temperature causes molecules to move faster, which raises the likelihood of collisions with sufficient energy to break bonds, thus increasing the rate of reaction. For every 10°C increase, the reaction rate typically doubles.
Why is the position of molecular collision important for reaction rates?
-The position of the collision matters because in larger molecules, only specific parts of the molecule (such as reactive groups) can participate in the reaction. Collisions that do not occur at these reactive sites will not lead to a successful reaction.
What role does concentration play in reaction rates?
-Increasing the concentration of reactant molecules raises the probability of collisions, thereby increasing the reaction rate. More molecules in a given space means a higher likelihood of successful interactions.
What is the difference between organic and inorganic reactions in terms of speed?
-Organic reactions typically occur more slowly than inorganic reactions because they involve larger, more complex molecules. The increased size leads to more specific and sometimes less frequent collisions at the reactive sites.
What does the law of mass action state about reaction rates?
-The law of mass action states that, at constant temperature and pressure, the rate of a reaction is directly proportional to the product of the concentrations of the reactants, each raised to a power equal to its coefficient in the balanced equation.
How do acids and bases differ in terms of their behavior in aqueous solutions?
-Acids donate protons (H+), while bases accept protons. In aqueous solutions, acids increase the concentration of H+ ions, whereas bases increase the concentration of OH- ions.
What is the significance of the dissociation coefficient (alpha) in acid and base strength?
-The dissociation coefficient (alpha) represents the fraction of molecules that dissociate into ions in solution. Strong acids and bases have a high dissociation coefficient, while weak acids and bases dissociate less, which is reflected in their strength.
How do we categorize acids based on their dissociation behavior?
-Acids are categorized as strong, moderate, or weak based on their dissociation in water. Strong acids dissociate completely (alpha > 90%), moderate acids dissociate partially (alpha between 5-50%), and weak acids dissociate minimally (alpha < 5%).
What does the term 'irreversible reaction' mean, and can you give an example?
-An irreversible reaction is one where the products cannot easily revert back to the reactants. An example is the combustion of gasoline, where CO2 and water are formed, and the process cannot be reversed to recover the original fuel.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Bioquímica - Aula 18 - Anabolismo

Bioquímica - Aula 15 - Catabolismo de Lipídios

085-Metabolic Pathways & Intermediates

Bioquímica - Aula 11 - Catabolismo - Carboidratos

VIDA E CARACTERÍSTICAS GERAIS DOS SERES VIVOS (PROVA, VESTIBULAR, ENEM) - OLHAR QUÍMICO |PROF. ROMEU
Bioquímica - Aula 03 - Alguns conceitos químicos importantes - 2
5.0 / 5 (0 votes)