Resumos dos Modelos Atômicos: Dalton, Thomson, Rutherford e Rutherford-Bohr!
Summary
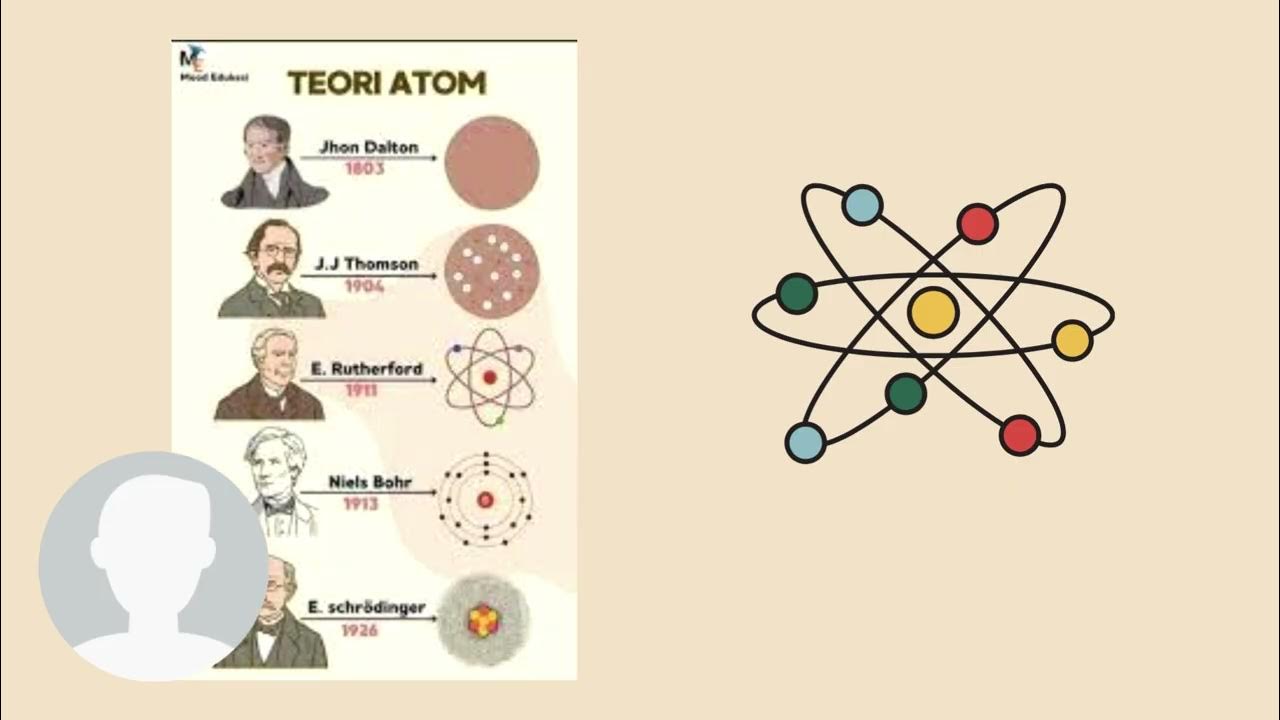
TLDRIn this video, the presenter provides an energetic overview of four major atomic models: Dalton, Thomson, Rutherford, and Bohr. The evolution of atomic theory is explored, from Dalton's indivisible atoms to Thomson's discovery of electrons and Rutherford's identification of the nucleus. Bohr's model introduces quantized energy levels for electrons. The video explains how each theory built upon the previous one, enhancing our understanding of the atom. Through lively commentary and engaging visuals, the presenter emphasizes the importance of studying these models, offering a fun and informative learning experience for viewers.
Takeaways
- 😀 The video introduces the four main atomic models in a fun and engaging way, helping viewers understand the evolution of the concept of the atom.
- 😀 The first model discussed is Dalton's atomic model, which proposed that atoms are indivisible, spherical, and indestructible particles that make up matter.
- 😀 Dalton's model also suggested that atoms combine, separate, and rearrange in chemical reactions without being created or destroyed.
- 😀 Thomson's atomic model followed, where he discovered electrons through cathode ray experiments and proposed that atoms are made of a positively charged sphere with embedded electrons.
- 😀 Thomson's model, known as the 'raisin pudding' model, explained the electric nature of materials, where electrons could be gained or lost, changing the atom's charge.
- 😀 Rutherford's model came next, based on his gold foil experiment, which revealed that atoms have a tiny, dense nucleus at the center, containing positive charge (protons) and neutral particles (neutrons).
- 😀 Rutherford’s model also identified that electrons orbit the nucleus in a vast space, making the nucleus incredibly small compared to the atom's overall size.
- 😀 Rutherford's atomic model is known as the 'planetary model' due to its resemblance to the solar system, where electrons orbit a central nucleus.
- 😀 Bohr improved upon Rutherford’s model by proposing that electrons move in fixed circular orbits or 'energy levels' around the nucleus, with quantized energy.
- 😀 Bohr’s model also explained atomic spectra, where electrons absorb or release energy when transitioning between orbits, creating light in specific wavelengths.
- 😀 The video concludes by mentioning further developments in atomic theory, such as energy sublevels, and encourages viewers to keep learning through the channel's content.
Q & A
What is the main focus of the video script?
-The main focus of the video script is explaining the evolution of atomic models in chemistry, highlighting four key atomic models: Dalton's, Thomson's, Rutherford's, and Rutherford-Bohr's models.
What is Dalton's atomic model known for?
-Dalton's atomic model is known for suggesting that matter is made up of indivisible and indestructible atoms, which are spherical and have a characteristic atomic weight. The model emphasized that chemical reactions involve only the combination, separation, and rearrangement of atoms.
Why was Dalton's atomic model limited in explaining certain phenomena?
-Dalton's atomic model was limited because it could not explain the conduction of electricity, which led to the development of new atomic models, such as Thomson's.
What contribution did J.J. Thomson make to atomic theory?
-J.J. Thomson discovered the electron and proposed the 'plum pudding' model, where atoms are seen as spheres of positive charge with electrons embedded in them, making the atom electrically neutral overall.
How did Rutherford's gold foil experiment challenge Thomson's model?
-Rutherford's gold foil experiment showed that most alpha particles passed through the gold foil, but some were deflected at large angles, suggesting that the atom has a small, dense, positively charged nucleus, leading to the rejection of Thomson's model.
What are the key features of Rutherford's atomic model?
-Rutherford's atomic model proposed that the atom consists of a small, dense, positively charged nucleus (containing protons and neutrons) surrounded by negatively charged electrons in an 'electrosphere'. This model is often called the 'planetary model' due to its similarity to the solar system.
What did Niels Bohr add to the atomic theory?
-Niels Bohr improved on Rutherford's model by suggesting that electrons move in circular orbits (or energy levels) around the nucleus, and these orbits have specific energy values. He also introduced the idea of quantized energy levels, where electrons can absorb or emit energy when transitioning between these levels.
What is meant by 'quantized energy' in Bohr's atomic model?
-In Bohr's model, 'quantized energy' means that electrons in an atom can only occupy certain discrete energy levels, and when they absorb or release energy, it is in specific amounts that correspond to the difference between these levels.
What is the significance of the atomic spectra in Bohr's model?
-The atomic spectra are important in Bohr's model because they result from electrons transitioning between energy levels. When an electron moves to a lower energy level, it emits energy in the form of light, creating the atomic spectrum, which Bohr used to explain the behavior of atoms.
How did the video emphasize the importance of studying atomic theory?
-The video emphasizes that atomic theory is essential for understanding chemistry and the world around us, as it is the foundation of life and chemical reactions. The video encourages viewers to appreciate the evolution of atomic models and their relevance to science and everyday life.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Modelos Atômicos I Dia 01 | Desafio de Carnaval

Struktur Atom - Perkembangan Model Atom Dalton, Thomson, Rutherford, Bohr, Mekanika Kuantum -Kimia X

GCSE Chemistry | History of the Atom

STRUKTUR ATOM : Perkembangan Teori Atom

STRUKTUR ATOM DAN SISTEM PERIODIK UNSUR : Struktur Atom - Materi KIMIA SMA Kelas 10, TKA SMA |Part 1
stuktur atom
5.0 / 5 (0 votes)