Deslocamento de Equilíbrio - Exercício Resolvido
Summary
TLDRThis video explains the industrial process for synthesizing ammonia through the Haber process, discussing the effects of pressure and temperature on equilibrium. High pressure favors ammonia formation due to fewer gas molecules on the product side, while high temperature typically shifts equilibrium away from ammonia due to its exothermic nature. Despite this, high temperatures are used in industry to speed up reaction rates. The process also uses a catalyst and ammonia is removed through liquefaction, further shifting equilibrium towards ammonia production. The video provides an in-depth look at why industrial conditions are optimized for both speed and yield in ammonia synthesis.
Takeaways
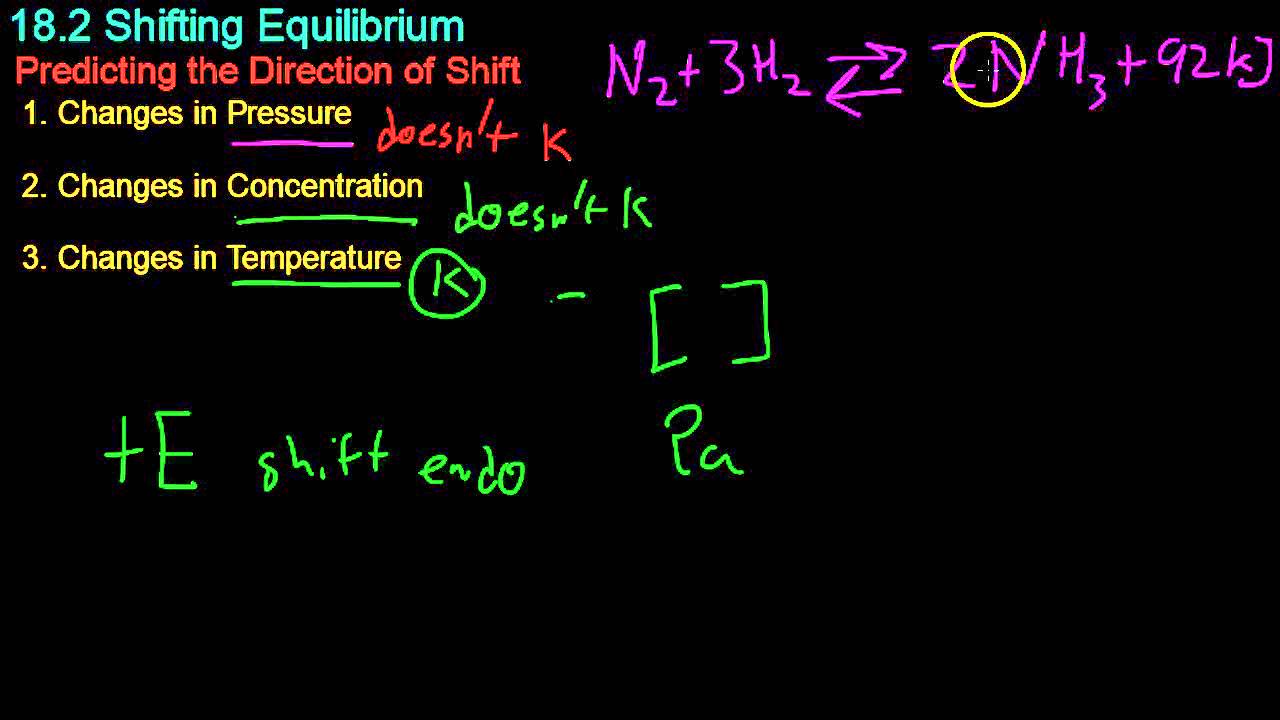
- 😀 High pressure favors ammonia production by shifting the equilibrium towards fewer moles of gas.
- 😀 Increasing temperature decreases the equilibrium constant for ammonia formation, favoring the reactants.
- 😀 The reaction for ammonia formation is exothermic, meaning it releases heat.
- 😀 At 25°C, the equilibrium constant (Kp) is 3.5 x 10⁹, while at 450°C, it drops significantly to 0.16.
- 😀 The reaction shifts toward ammonia production when pressure is increased because it reduces the volume of gas in the system.
- 😀 Higher temperatures, while lowering equilibrium constant, are required to speed up the reaction rate.
- 😀 Industrial conditions use high pressure (300-400 atm) and temperature (450°C) to balance equilibrium and reaction speed.
- 😀 A catalyst, typically iron, is used to speed up the reaction without affecting the equilibrium.
- 😀 Ammonia, a liquid at -33°C, can be easily condensed from its gaseous form, aiding in the separation and removal from the reaction mixture.
- 😀 The industrial process utilizes liquefaction to continuously remove ammonia, shifting the equilibrium to favor more ammonia production.
Q & A
What is the chemical equation for the ammonia formation reaction?
-The chemical equation for the ammonia formation reaction is: N2 (g) + 3H2 (g) ⇌ 2NH3 (g).
How does increasing the temperature affect the equilibrium constant (Kp) for ammonia production?
-Increasing the temperature decreases the equilibrium constant (Kp). For example, at 25°C, Kp is 3.5 × 10⁹, while at 450°C, it drops to 0.16, indicating that the reaction favors the reactants at higher temperatures.
What principle explains how pressure affects the ammonia formation equilibrium?
-Le Chatelier's Principle explains that increasing the pressure will shift the equilibrium towards the side with fewer moles of gas. In the ammonia formation reaction, increasing the pressure favors the production of ammonia (NH3), since there are fewer moles of gas on the product side (2 moles) compared to the reactant side (4 moles).
Why does increasing pressure favor the formation of ammonia in this reaction?
-Increasing pressure favors the formation of ammonia because the product side of the reaction has fewer moles of gas (2 moles of NH3) compared to the reactant side (4 moles: 1 mole of N2 and 3 moles of H2). According to Le Chatelier's Principle, the system will shift towards the side with fewer gas molecules to counteract the pressure increase.
What role does temperature play in the industrial synthesis of ammonia?
-In industrial ammonia synthesis, high temperatures (450°C) are used to increase the reaction rate, despite the fact that higher temperatures decrease the equilibrium constant. The elevated temperature helps the reaction proceed faster, although it slightly reduces ammonia yield due to the shift in equilibrium towards the reactants.
Why is a catalyst, such as iron, used in the ammonia production process?
-A catalyst, like iron, is used in the ammonia production process to speed up the reaction without affecting the equilibrium. It helps to lower the activation energy, enabling the reaction to proceed more quickly, which is crucial for efficient industrial production.
How does ammonia removal contribute to the production process?
-Ammonia removal is crucial because it drives the equilibrium towards the production of more ammonia. As ammonia is condensed and removed from the system, the reaction shifts to replace the lost ammonia, ensuring that more ammonia is produced continuously.
What is the significance of the temperatures of ammonia, nitrogen, and hydrogen in the condensation process?
-The ammonia, nitrogen, and hydrogen gases have significantly different boiling points, which aids in the condensation process. Ammonia has a boiling point of -33°C, nitrogen has -196°C, and hydrogen has -253°C. Ammonia condenses at -33°C, while nitrogen and hydrogen remain in the gaseous state, allowing ammonia to be separated efficiently.
Why is high temperature (450-500°C) still used in the ammonia synthesis process despite the decrease in ammonia yield?
-High temperatures are used in ammonia synthesis to increase the reaction rate. While they decrease the equilibrium constant and reduce the yield of ammonia, they are necessary to achieve an industrially viable reaction speed. Without elevated temperatures, the reaction would proceed too slowly.
What industrial conditions are typically used for ammonia production, and why?
-Ammonia production typically uses pressures between 300 and 400 atmospheres and temperatures of 450-500°C. These conditions favor the formation of ammonia due to the pressure shift towards the product side and the high temperature required to increase the reaction rate. Additionally, the use of a catalyst (like iron) ensures faster reaction kinetics.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Faktor-Faktor yang Mempengaruhi Pergeseran Kesetimbangan dan Penerapannya dalam Industri

"DESLOCAMENTO" DE EQUILÍBRIO - Princípio de Le Chatelier
18.2 Shifting Equilibrium

PEMBUATAN AMONIA || HABER-BOSCH PROCES

6. Chemical Reactions (Part 4) (4/5) (Cambridge IGCSE Chemistry 0620 for 2023, 2024 & 2025)

Equilibrium: Crash Course Chemistry #28
5.0 / 5 (0 votes)