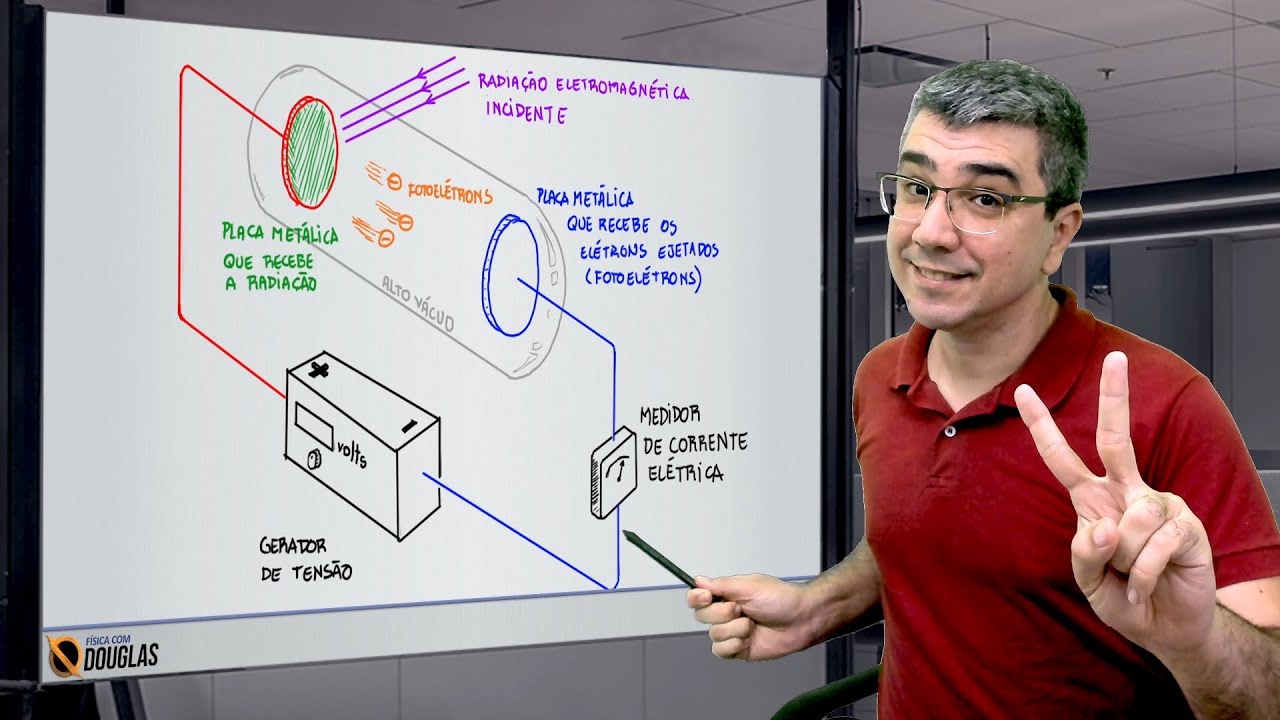
Efeito Fotoelétrico - O Nobel de Einstein
Summary
TLDRThis script explores the groundbreaking discovery of the photoelectric effect, which challenged classical wave theory and supported quantum theory. Hertz’s experiments revealed that ultraviolet radiation could eject electrons from a metal plate, but classical theory couldn't explain the results. Quantum theory, introduced by Einstein in 1905, resolved the issue by explaining that light consists of quantized packets of energy (photons). The energy of a photon depends on frequency, not intensity, which explains the instantaneous ejection of electrons and their kinetic energy. The theory was later experimentally confirmed, earning Einstein the Nobel Prize in Physics in 1921.
Takeaways
- 😀 The photoelectric effect is a phenomenon where electrons are ejected from a metal surface when exposed to light or radiation, challenging classical theories of physics.
- 😀 Heinrich Hertz first observed the photoelectric effect, but it was Albert Einstein’s work that explained the phenomenon through quantum mechanics.
- 😀 Classical wave theory, as proposed by James Maxwell, failed to explain key aspects of the photoelectric effect, such as the instantaneous ejection of electrons.
- 😀 According to classical theory, light's intensity was believed to be proportional to its energy, but this did not align with experimental results showing that light of low intensity but high frequency could still eject electrons.
- 😀 Einstein proposed that light is made up of discrete energy packets called photons, with the energy of each photon determined by the light's frequency, not its intensity.
- 😀 The photoelectric effect demonstrated that light behaves both as a wave and as a particle, a concept now known as wave-particle duality.
- 😀 Einstein’s quantum theory explained that the energy of each photon is what determines whether an electron is ejected, not the intensity of the light.
- 😀 Light of low frequency could not eject electrons because the photons did not carry enough energy, regardless of light intensity, while high-frequency light (even at low intensity) could knock electrons loose.
- 😀 The photoelectric effect was an important puzzle for classical physics that quantum theory resolved, providing a clear explanation of how light interacts with matter.
- 😀 Albert Einstein received the Nobel Prize in Physics in 1921, not for his theory of relativity, but for his explanation of the photoelectric effect, which helped establish quantum mechanics as a fundamental theory in physics.
Q & A
What discovery challenged classical electromagnetic wave theory?
-The discovery of the photoelectric effect, where light striking a metallic plate ejected electrons, challenged classical electromagnetic wave theory.
How did Heinrich Hertz's experiment contribute to the understanding of the photoelectric effect?
-Hertz discovered that ultraviolet radiation could cause electrons to be ejected from a metal plate, but his findings were inconsistent with classical wave theory, which led to further investigations into the phenomenon.
What discrepancy between classical and quantum theories did the photoelectric effect reveal?
-The discrepancy was that classical theory suggested the energy of ejected electrons should depend on the intensity of the light, but experimental results showed it depended on the frequency, which was explained by quantum theory instead.
What is the key difference between classical wave theory and quantum theory in explaining the photoelectric effect?
-Classical wave theory explained light as a continuous wave, where energy was determined by intensity, while quantum theory introduced the concept of quantized photons, where the energy of each photon depends on its frequency.
How did Einstein's interpretation of the photoelectric effect differ from earlier theories?
-Einstein proposed that light consisted of quantized photons, where each photon had energy proportional to its frequency, explaining why only light of certain frequencies could eject electrons and why the process was instantaneous.
Why did the photoelectric effect occur instantaneously according to Einstein’s theory?
-Einstein’s theory suggested that the interaction between photons and electrons was nearly instantaneous because each photon transferred a specific amount of energy to an electron, allowing it to be ejected immediately.
How did Robert Millikan's experiments confirm Einstein's theory?
-Millikan conducted extensive experiments that validated the predictions made by Einstein's photoelectric effect theory, particularly the relationship between the frequency of light and the energy of ejected electrons.
What did the intensity of light affect in the photoelectric effect experiment?
-In the photoelectric effect, the intensity of light affected the number of electrons ejected, not their energy. Higher intensity meant more photons, and thus more electrons were ejected, but the energy of each electron was determined by the frequency of the light.
Why was it significant that light of low frequency could not eject electrons in the photoelectric effect?
-This was significant because it contradicted the classical theory, which predicted that light of any frequency could eject electrons if the intensity was high enough. The quantum explanation showed that only light above a certain frequency could have enough energy per photon to eject electrons.
What key achievement earned Einstein the Nobel Prize in Physics in 1921?
-Einstein was awarded the Nobel Prize in 1921 for his explanation of the photoelectric effect, which applied quantum theory to explain the behavior of light and electrons, a major contribution to the development of quantum mechanics.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)