16.27 | By calculating ΔSuniv at each temperature, determine if the melting of 1 mole of NaCl(s) is
Summary
TLDRThis video tutorial explains how to determine whether the melting of NaCl (sodium chloride) is spontaneous at 500°C and 700°C by calculating the entropy change of the universe. Using the formula for ΔS_universe, the process considers entropy changes in the system and surroundings, incorporating values for entropy of NaCl solid and liquid, as well as the enthalpy of fusion. The video walks through each step of the calculation and concludes that the melting process is non-spontaneous at both temperatures, providing key assumptions about the thermodynamic values used in the analysis.
Takeaways
- 😀 The task involves determining whether the melting of NaCl solid to NaCl liquid is spontaneous at 500°C and 700°C by calculating the entropy change (ΔS) for the universe.
- 😀 To solve the problem, we use the equation ΔS_universe = ΔS_system + q/T, where ΔS_system is the change in entropy of the system, q is the heat exchanged, and T is the temperature in Kelvin.
- 😀 The entropy values for NaCl solid and liquid are provided as 72.11 J/mol·K and 95.06 J/mol·K, respectively.
- 😀 The enthalpy of fusion (ΔH fusion) is given as 27.95 kJ/mol, which is converted to 27,950 J/mol to match the entropy units in joules.
- 😀 The temperatures 500°C and 700°C are converted to Kelvin (773 K and 973 K, respectively) to maintain consistent units.
- 😀 The change in entropy for the system (ΔS_system) is calculated by subtracting the entropy of NaCl solid from the entropy of NaCl liquid, resulting in 22.95 J/mol·K.
- 😀 The heat value (q) for the system is negative when considering the surroundings (ΔH fusion), since the surroundings' heat is the inverse of the system's heat value.
- 😀 For both 500°C and 700°C, the ΔS_universe values are calculated and found to be negative, indicating that the process is not spontaneous at these temperatures.
- 😀 At 500°C, the ΔS_universe is -13.2 J/mol·K, and at 700°C, the ΔS_universe is -5.8 J/mol·K, confirming that neither temperature results in a spontaneous process.
- 😀 The assumptions made in the problem include that entropy values do not change significantly across the different temperatures (298 K vs 773 K and 973 K), and that the enthalpy values are treated as constants for the system.
- 😀 The overall conclusion is that the melting of NaCl at both 500°C and 700°C is not spontaneous, as the entropy change for the universe is negative in both cases.
Q & A
What is the main task of the video script?
-The main task is to determine if the melting of one mole of NaCl solid is spontaneous at 500°C and 700°C by calculating the change in entropy (ΔS) for the universe at these temperatures.
What is the formula used to calculate the change in entropy for the universe?
-The formula used is: ΔS_universe = ΔS_system + q_surroundings / T, where ΔS_system is the change in entropy of the system, q_surroundings is the heat exchanged with the surroundings, and T is the temperature in Kelvin.
What values are given in the script to solve the problem?
-The given values are: entropy of NaCl solid (72.11 J/mol·K), entropy of NaCl liquid (95.06 J/mol·K), and the enthalpy of fusion (ΔH_fusion) of NaCl (27.95 kJ/mol).
Why do we convert the enthalpy of fusion (ΔH_fusion) to joules per mole?
-The enthalpy of fusion is provided in kilojoules, but the entropy values are given in joules per mole·K. To maintain consistent units, we convert the enthalpy of fusion from kilojoules to joules by multiplying by 1,000.
What is the significance of the change in entropy for the system (ΔS_system)?
-The change in entropy for the system, ΔS_system, is calculated as the difference between the entropy of the products (NaCl liquid) and the reactants (NaCl solid). This value helps determine the overall entropy change in the system as NaCl transitions from solid to liquid.
How do we determine if the melting of NaCl is spontaneous at a given temperature?
-The process is spontaneous if the change in entropy for the universe (ΔS_universe) is positive. If ΔS_universe is negative, the process is non-spontaneous. For both 500°C and 700°C, ΔS_universe turns out to be negative, indicating that the process is non-spontaneous at both temperatures.
What does a negative value of ΔS_universe indicate?
-A negative value of ΔS_universe indicates that the process is non-spontaneous, meaning that the universe is moving toward a state of less disorder or randomness.
Why is the q_surroundings term in the formula for ΔS_universe negative?
-The q_surroundings term is negative because the heat released by the system during the fusion process (heat absorbed by the surroundings) is considered negative when calculating the surroundings' entropy change.
What assumptions are made regarding entropy and enthalpy values used in the calculations?
-It is assumed that the entropy and enthalpy values remain constant over the temperature range of 500°C to 700°C, despite the fact that they were initially measured at a standard temperature of 298.15 K.
How does the temperature affect the calculation of ΔS_universe?
-The temperature affects the denominator in the q_surroundings / T term of the ΔS_universe formula. As the temperature increases (from 773 K at 500°C to 973 K at 700°C), the value of ΔS_universe becomes less negative, but it still remains negative, indicating non-spontaneity.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Reactivo Limitante y en Exceso (Paso a Paso)
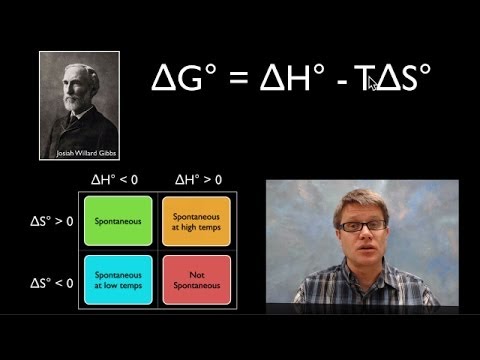
Using Gibbs Free Energy

Misturas de soluções de solutos diferentes sem reação - Aula 09 | #soluções #youtubeedu

LENGKAP ‼️ CARA MENGHITUNG pH HIDROLISIS GARAM - KELAS 11

Tata Nama Senyawa Kimia | Senyawa Biner | Kimia kelas 10

Ikatan Kimia Animasi | Ikatan Ion | Pembentukan Natrium Klorida
5.0 / 5 (0 votes)