Video 2 1080p
Summary
TLDRThis video explores the key types of fluids used in medical prescriptions: 0.9% Sodium Chloride, Hartmann's Solution, and 5% Glucose. It delves into their compositions, including electrolyte content, and how these fluids resemble the body’s extracellular fluid. The video also explains how to choose the appropriate fluid based on the patient’s condition, highlighting the risks and complications that may arise, such as acidosis or hyperkalemia. Additionally, it covers how fluids are distributed within the body and the specific situations where each fluid type is most beneficial.
Takeaways
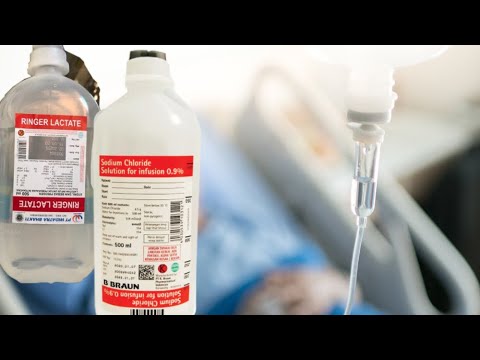
- 😀 Fluid prescribing includes three main options: 0.9% Sodium Chloride, Hartmann's Solution (Compound Sodium Lactate), and 5% Glucose.
- 😀 The percentage in fluid concentrations indicates the amount of solute (e.g., 0.9% means 9 grams of sodium chloride per liter).
- 😀 Hartmann's Solution contains sodium, chloride, potassium, and calcium, which can be important for managing electrolyte imbalances.
- 😀 The pH of prescribed fluids is not a major concern as they are buffered within the body, diluting during metabolism.
- 😀 Sodium Chloride and Hartmann’s Solution are most similar to extracellular fluid, especially blood plasma, which makes them ideal for conditions like hypovolemia.
- 😀 Key differences between sodium chloride and Hartmann’s include higher chloride content in sodium chloride and lactate in Hartmann’s.
- 😀 Sodium Chloride is the most commonly prescribed fluid due to its availability and broad use in various clinical situations.
- 😀 Patient conditions should be considered when prescribing fluids, such as avoiding excessive sodium chloride or Hartmann’s in heart failure.
- 😀 Severe liver disease affects lactate metabolism, meaning Hartmann's Solution may worsen lactic acidosis in these patients.
- 😀 5% Glucose solution, although useful for regular maintenance, is not ideal for fluid resuscitation because it distributes water throughout both intracellular and extracellular compartments.
Q & A
What are the three main fluids available to prescribe to patients?
-The three main fluids available for prescription are 0.9% sodium chloride, Hartmann's solution (compound sodium lactate), and 5% glucose solution.
What does the percentage in a fluid solution represent?
-The percentage in a fluid solution represents the concentration of the solute in the solution. For example, 0.9% sodium chloride means there are 9 grams of sodium chloride dissolved in one liter of liquid.
What electrolytes are found in Hartmann's solution?
-Hartmann's solution contains sodium, chloride, potassium, and calcium. It also contains lactate, which is important in certain medical conditions.
How do sodium chloride and Hartmann's solution compare to the body's fluids?
-Both sodium chloride and Hartmann's solution closely resemble extracellular fluid, particularly blood plasma. However, sodium chloride has a higher chloride concentration, and Hartmann's contains lactate, which can affect the body's acid-base balance.
Why is 0.9% sodium chloride the most commonly prescribed fluid?
-0.9% sodium chloride is the most widely available and preferred solution for many medical conditions due to its resemblance to extracellular fluid, making it effective in treating various forms of hypovolemia and dehydration.
What complications can arise from prescribing too much sodium chloride or Hartmann's solution?
-Prescribing too much sodium chloride or Hartmann's solution can worsen conditions like heart failure, where the heart struggles to pump blood, and cause fluid retention, increasing the risk of complications.
Why should Hartmann's solution be avoided in patients with severe liver disease?
-Hartmann's solution contains lactate, which is normally metabolized by the liver to form bicarbonate. In patients with severe liver disease, this process is impaired, potentially exacerbating lactic acidosis.
How can sodium chloride lead to acidosis?
-Sodium chloride can lead to acidosis because the chloride content increases in the blood, causing a reduction in bicarbonate levels. This imbalance results in a state of acidosis.
What is the main limitation of 5% glucose solution in fluid resuscitation?
-The main limitation of 5% glucose solution in fluid resuscitation is that once metabolized, the glucose is used for energy, and the water is distributed freely between intracellular and extracellular compartments, reducing its effectiveness for resuscitation.
What factors should be considered when choosing a fluid to prescribe to a patient?
-When choosing a fluid, consider the patient's existing conditions, such as heart failure, liver disease, or renal disease, as well as the potential complications the fluid may cause, like acidosis or electrolyte imbalances.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
Informasi Berbagai Jenis Cairan Infus, Indikasi Medis dan Kegunaannya

IV Fluids

Terapi cairan infus ( RL, NaCl, Dextrose, Albumin, Dextran, Gelatin, HES, KCL, Kabiven)

Cara Cepat Infus/Terapi Cairan di IGD | Fluid Therapy in Emergency Settings Made Incredibly Easy

DIFFERENCE BETWEEN NEWTONIAN AND NON-NEWTONIAN FLUIDS

ADVANCED MEDICAL VOCABULARY 💊 | Words & Phrases You Should Know
5.0 / 5 (0 votes)