Modelos atómicos (Dalton, Thomson, Rutherford, Bohr y Chadwick)
Summary
TLDRThis video script provides a historical overview of atomic theory, starting with Democritus' concept of indivisible 'atoms' and progressing through significant scientific discoveries. In 1808, John Dalton defined atoms as the smallest units of elements, identical in size, mass, and chemical properties. Dalton's atomic theory laid the groundwork for understanding that different elements have distinct properties due to variations in their atomic structure. The script then moves to Joseph Thomson's discovery of the electron in 1897, leading to the 'plum pudding' model of the atom, where electrons are scattered throughout a sphere of positive charge. However, Ernest Rutherford's gold foil experiment in 1909 revealed a small, positively charged nucleus with electrons orbiting around it, which led to the planetary model of the atom. Niels Bohr further refined the model in 1913 by introducing quantized energy levels for electrons, explaining atomic stability and the emission of photons when electrons transition between these levels. Bohr's work earned him the Nobel Prize in Physics in 1922 and addressed the mystery of atomic weights. The discovery of the neutron by James Chadwick in 1932 completed the picture of the atom, with the neutron being a subatomic particle with no electric charge and slightly more mass than the proton. Chadwick's discovery was honored with the Nobel Prize in Physics in 1935 and explained why helium, with two protons, weighed four times more than hydrogen with one proton. The video concludes by emphasizing how these atomic models and discoveries have contributed to our current understanding of the atom, highlighting the contributions of each scientist and the significance of their findings in shaping modern atomic theory.
Takeaways
- 🤔 Democritus, one of the first philosophers, questioned the nature of matter and proposed the concept of the atom as the smallest indivisible particle.
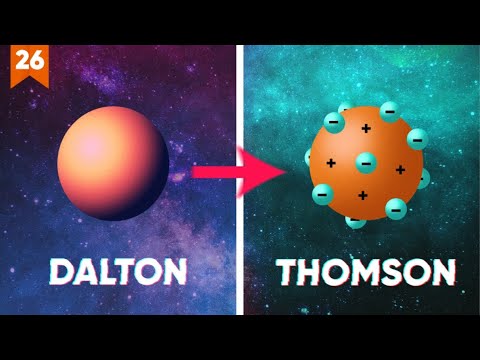
- 🔬 In 1808, John Dalton defined atoms more precisely as small, indivisible, and indestructible particles that form elements, with all atoms of the same element being identical in size, mass, and chemical properties.
- 🌐 Dalton's atomic theory stated that chemical compounds are formed by the union of atoms of different elements in fixed proportions, with a whole number ratio.
- 🚫 Chemical reactions only involve the separation, combination, or reordering of atoms, without creating or destroying them.
- 🔋 In 1897, J.J. Thomson discovered the electron, a negatively charged particle, leading to a new model of the atom as a sphere of positive electricity with electrons embedded within.
- ⚛️ Ernest Rutherford's gold foil experiment in 1909 revealed that atoms have a mostly empty space with a small, positively charged nucleus, where most of the atom's mass is concentrated.
- ☀️ In 1913, Niels Bohr proposed a planetary model of the atom, suggesting that electrons orbit the nucleus in specific energy levels and can only occupy certain allowed orbits.
- 🏆 Rutherford was awarded the Nobel Prize in Physics in 1908 for his research on the atomic nucleus, and Bohr in 1922 for his contribution to understanding atomic behavior and quantum mechanics.
- 🤔 The problem of atomic weight was unresolved until James Chadwick discovered the neutron in 1932, a subatomic particle with no electric charge and slightly more mass than a proton.
- 🏆 Chadwick's discovery of the neutron earned him the Nobel Prize in Physics in 1935, and it helped explain why heavier elements like helium have more mass than hydrogen, despite having a similar number of protons.
- 🧬 Collectively, these discoveries and models by Dalton, Thomson, Rutherford, Bohr, and Chadwick laid the foundation for our current understanding of the atom.
Q & A
Who is considered one of the first philosophers to question the nature of matter?
-Democritus is considered one of the first philosophers to question the nature of matter, proposing the concept of the atom as an indivisible particle.
What was John Dalton's contribution to the atomic theory?
-John Dalton defined the atom more precisely, proposing that elements are made of small, indivisible, and indestructible particles called atoms, which are identical in size, mass, and chemical properties within the same element.
What was the significance of Joseph Thomson's discovery in atomic theory?
-Joseph Thomson discovered the electron, which led to the understanding that atoms are not indivisible and are composed of smaller particles. He proposed a 'plum pudding' model where electrons are embedded within a sphere of positive charge.
What did Ernest Rutherford's gold foil experiment reveal about the atomic structure?
-Ernest Rutherford's gold foil experiment revealed that atoms have a significant amount of empty space and a small, dense, positively charged nucleus, which led to the proposal of a new atomic model with electrons orbiting around the nucleus.
What was Niels Bohr's contribution to the understanding of atomic structure?
-Niels Bohr developed a planetary model of the atom, suggesting that electrons can only occupy specific energy levels and that an electron can move to a higher energy level by absorbing radiation, and emit energy as a photon when it returns to its original orbit.
Who discovered the neutron and how did this discovery impact the atomic model?
-James Chadwick discovered the neutron, a subatomic particle with no electric charge and slightly more mass than a proton. This discovery helped to explain the mass of atoms, as the neutron contributes significantly to the overall mass, which was previously unaccounted for.
How did the discovery of the electron, proton, and neutron contribute to the modern understanding of the atom?
-The discovery of the electron by Thomson, the proton by Rutherford, and the neutron by Chadwick, along with the development of various atomic models, provided a comprehensive understanding of the atom's structure, with a positively charged nucleus containing protons and neutrons, and electrons orbiting around it.
What is the significance of the atomic number in relation to energy levels?
-The atomic number signifies the number of protons in the nucleus of an atom and also determines the number of electrons in a neutral atom. It is directly related to the energy levels, as each energy level can hold a specific number of electrons, which is crucial for understanding chemical behavior.
null
-null
How did the discovery of subatomic particles lead to the development of quantum mechanics?
-The discovery of subatomic particles like electrons, protons, and neutrons, along with the understanding of their behavior in atoms, laid the groundwork for quantum mechanics. This field of physics describes the behavior of matter and energy at the atomic and subatomic level, where classical physics no longer applies.
What is the role of chemical compounds in the context of atomic theory?
-Chemical compounds are formed when two or more atoms of different elements combine in fixed proportions. The understanding of atomic theory explains how these compounds are formed through the sharing or transfer of electrons between atoms, leading to stable molecules with specific properties.
How did the atomic theory evolve from Democritus to the modern understanding of the atom?
-The atomic theory evolved from Democritus's concept of indivisible atoms to Dalton's definition of elements as made of identical atoms, through Thomson's discovery of the electron, Rutherford's discovery of the nucleus, Bohr's planetary model with quantized energy levels, and finally, Chadwick's discovery of the neutron, leading to the modern understanding of the atom as a complex system of subatomic particles.
What are the implications of atomic theory for the field of chemistry?
-Atomic theory is fundamental to chemistry as it explains the nature of elements, their reactivity, and the formation of chemical bonds. Understanding the structure and properties of atoms allows chemists to predict how different substances will interact, form compounds, and participate in chemical reactions.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Development of Atomic Theory: An Introduction

GCSE Chemistry | History of the Atom

PERKEMBANGAN MODEL ATOM ( DALTON, THOMSON, RUTHERFORD, NIELS BOHR, MEKANIKA KUANTUM)
O Modelo Atômico de Dalton x Thomson

History of the Atom (Atomic Theory)

Quarter 2_WEEK 1 - DAY 1 DALTON AND DEMOCRITUS | Science 8 MATATAG Curriculum
5.0 / 5 (0 votes)