GCSE Chemistry Revision "Cells and Batteries" (Triple)
Summary
TLDRThis video explains the fundamentals of cells and batteries, particularly for chemistry students. It covers how a cell generates electricity by placing two different metals in an electrolyte and connecting them to a voltmeter. The potential difference between the metals is influenced by their reactivity. A battery is simply two or more cells connected in series to produce a greater voltage. The video also explores the differences between rechargeable and non-rechargeable batteries, focusing on how chemical reactions in rechargeable batteries can be reversed, unlike in non-rechargeable ones.
Takeaways
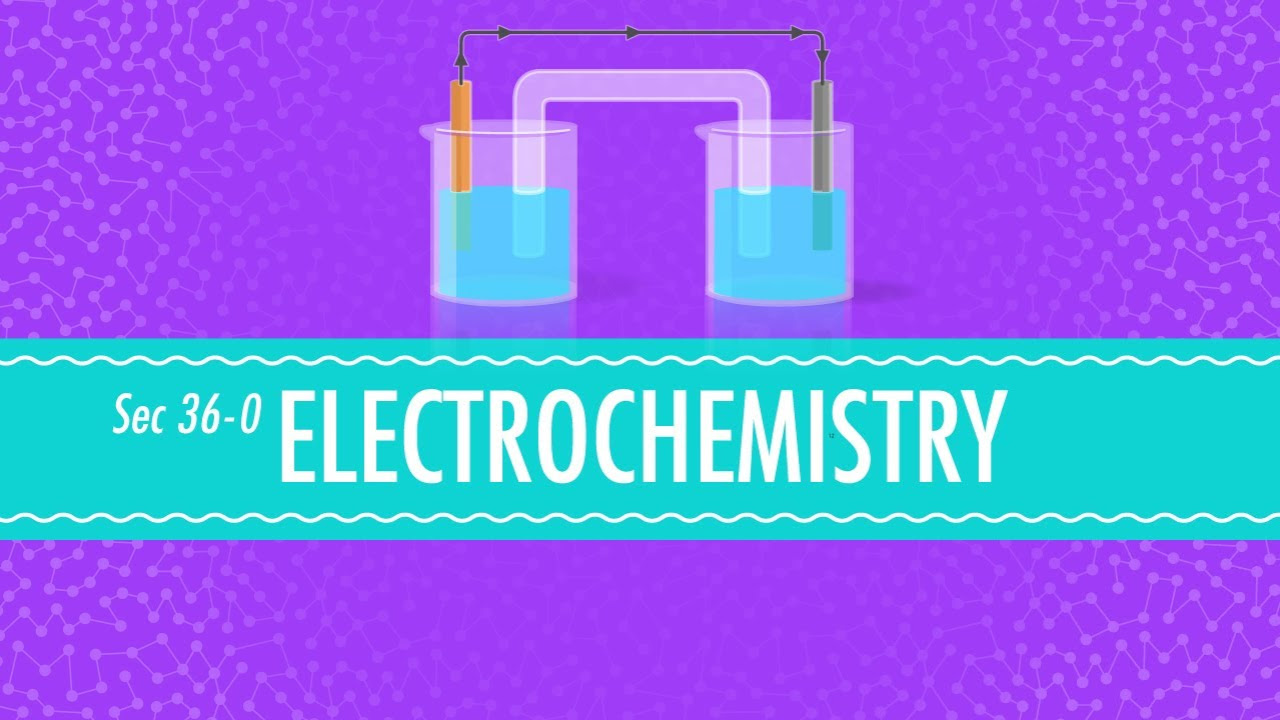
- 🔋 **Electricity from Chemistry**: A cell generates electricity when two different metals are placed in an electrolyte, which is a conductive solution.
- 🔬 **Chemical Reactions**: The electricity is produced by chemical reactions occurring on the surface of the metals in the cell.
- ⏲️ **Limited Lifespan**: Cells can only produce electricity for a certain period as the chemicals within them eventually deplete.
- 📊 **Reactivity Series**: The potential difference, or voltage, produced by a cell depends on the reactivity difference between the two metals used.
- 🏆 **Top Metals**: Reactive metals like magnesium and aluminum are at the top of the reactivity series, producing higher potential differences when used in cells.
- 🥈 **Less Reactive Metals**: Less reactive metals such as lead and copper are at the bottom of the series and produce lower potential differences.
- 🔌 **Electrolyte's Role**: The electrolyte also affects the potential difference, although the details are not specified in the script.
- 🔋 **Batteries Defined**: A battery contains two or more cells connected in series, which increases the total voltage produced.
- 🔁 **Rechargeable vs. Non-Rechargeable**: Non-rechargeable batteries cannot have their chemical reactions reversed, unlike rechargeable batteries which can be recharged by applying electrical current.
- 🔄 **Reversible Reactions**: Rechargeable batteries allow for the reversal of chemical reactions, which is not possible with non-rechargeable batteries once they're depleted.
Q & A
What is a cell in terms of electrical chemistry?
-A cell in electrical chemistry is a setup where two different metals are placed into an electrolyte solution. This chemical reaction between the metals generates electricity, which can be measured as a potential difference or voltage.
How does an electrolyte contribute to electricity production in a cell?
-An electrolyte is a solution that can conduct electricity, allowing ions to move between the two metals in the cell. This movement facilitates the chemical reaction that produces electricity.
What are the two factors that affect the potential difference in a cell?
-The potential difference in a cell depends on two main factors: the difference in reactivity between the two metals used and the electrolyte solution.
Why can't cells produce electricity indefinitely?
-Cells cannot produce electricity indefinitely because the chemicals involved in the reactions eventually run out. Once the reactions stop, no more electricity is produced.
What is the relationship between metal reactivity and potential difference in a cell?
-The greater the difference in reactivity between the two metals in a cell, the larger the potential difference (voltage) produced. For example, a combination of magnesium and copper produces a higher potential difference than zinc and tin.
How is a battery different from a cell?
-A battery is made up of two or more cells connected in series, producing a greater overall voltage than a single cell.
What is the main difference between rechargeable and non-rechargeable batteries?
-The main difference is that rechargeable batteries can be recharged by reversing the chemical reactions using an external electrical current, while non-rechargeable batteries cannot be recharged because the chemical reactions are irreversible.
What role does the electrolyte play in determining the potential difference?
-The electrolyte in a cell also affects the potential difference, although the details of this are not covered in depth. The electrolyte facilitates the ion movement that allows the chemical reactions to produce electricity.
Why does connecting cells in series increase the voltage in a battery?
-When cells are connected in series, their voltages add up, resulting in a greater overall potential difference. For example, two cells of 2.5 volts each connected in series produce a total of 5 volts.
What is the reactivity series, and how does it relate to cell design?
-The reactivity series ranks metals by their reactivity, with more reactive metals like magnesium at the top and less reactive metals like copper at the bottom. In a cell, the difference in reactivity between the two metals used determines the size of the potential difference produced.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)