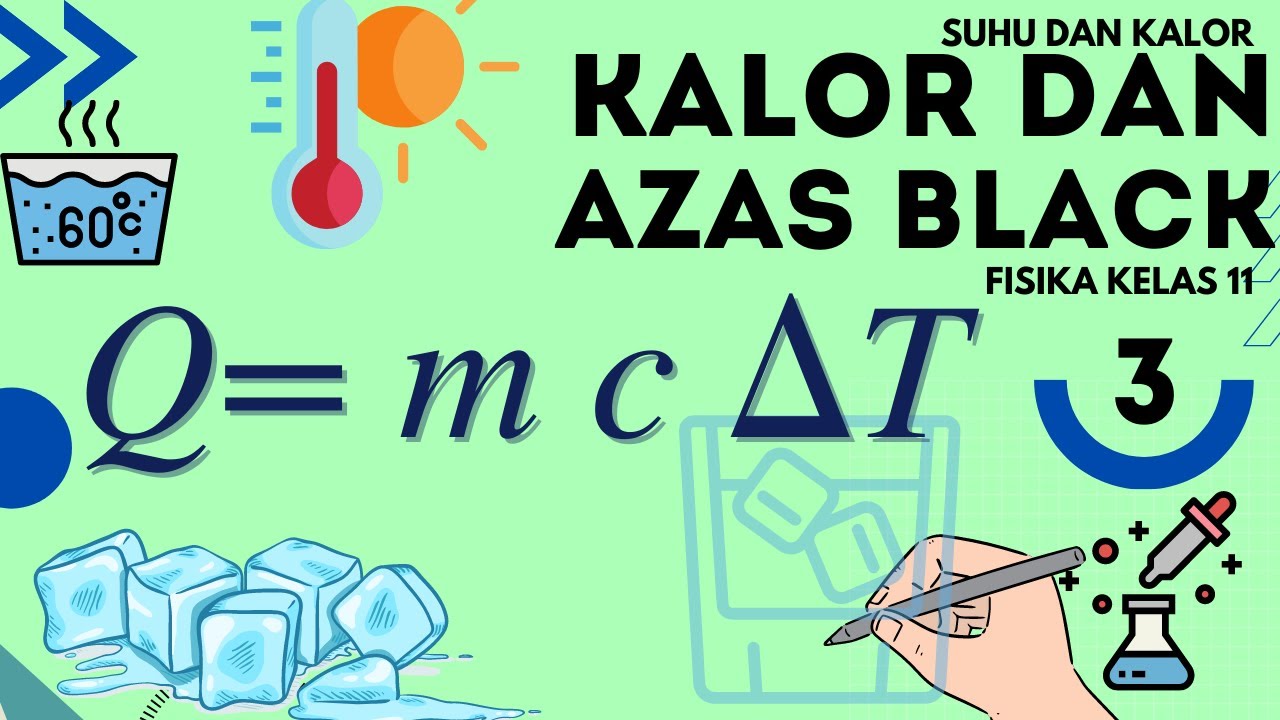Which Has More Energy an Iceberg or a Cup of Coffee
Summary
TLDRThis video explores the differences between thermal energy, temperature, and heat energy. Using examples like a cup of hot coffee and an iceberg, it demonstrates how thermal energy depends on mass and size, while temperature measures average kinetic energy. The video also explains how heat energy moves from warmer to cooler objects, with an example of pouring coffee onto an iceberg. Additionally, the conductive properties of materials, like aluminum versus a paperback book, are examined to show how they impact the sensation of temperature. Understanding these concepts is key to grasping broader physics and chemistry principles.
Takeaways
- 😀 Thermal energy refers to the total kinetic energy of molecules in a system, and is dependent on the size and mass of the object.
- 😀 Temperature measures the average kinetic energy of molecules in a substance and is not influenced by size or mass.
- 😀 Thermal energy is measured in **Joules**, while temperature is measured in **degrees Celsius or Fahrenheit**.
- 😀 A cup of hot coffee does not have more thermal energy than an iceberg, even though it may have a higher temperature, because the iceberg is much larger in mass.
- 😀 When comparing one cup of coffee to two cups, the temperature remains the same, but the thermal energy doubles due to the increased mass.
- 😀 An iceberg contains more thermal energy than a cup of coffee, even if it is at a lower temperature, due to its significantly larger mass.
- 😀 Heat energy moves from an object with higher kinetic energy to one with lower kinetic energy until equilibrium is reached.
- 😀 If coffee is poured onto an iceberg, the coffee will lose energy, cooling down, while the iceberg will barely warm up due to its large mass.
- 😀 Heat transfer is affected by the physical and chemical properties of materials, such as their **conductivity**.
- 😀 A material like aluminum feels colder than a paperback book at the same temperature because it conducts heat away from your hand more quickly, even though both are at the same temperature.
Q & A
What is the common misconception about thermal energy in relation to an iceberg and a cup of hot coffee?
-Many people assume that a cup of hot coffee has more thermal energy than a massive iceberg because of the temperature difference, but thermal energy also depends on mass, not just temperature. The iceberg, due to its larger mass, actually has more thermal energy than the cup of coffee.
How do thermal energy and temperature differ?
-Thermal energy refers to the total amount of kinetic energy of the molecules in a system, and it is impacted by the system's mass and size. Temperature, however, measures the average kinetic energy of molecules, and it is not affected by mass or size.
What happens when two cups of coffee are compared to one cup of coffee in terms of temperature and thermal energy?
-Two cups of coffee will not have double the temperature of one cup, because temperature measures average kinetic energy, which doesn't double with mass. However, the two cups will have double the thermal energy because thermal energy is proportional to mass.
Why does the iceberg contain more thermal energy than a cup of hot coffee despite its lower temperature?
-The iceberg contains more thermal energy than the cup of coffee because its mass is much greater. Although the temperature of the iceberg is lower, thermal energy depends not only on temperature but also on the size or mass of the object.
What is heat energy and how does it transfer between objects?
-Heat energy is the transfer of energy between two systems with different kinetic energies. It always moves from the system with higher kinetic energy to the one with lower kinetic energy, until thermal equilibrium is reached.
If hot coffee is poured onto an iceberg, which will cool off and which will warm up?
-The hot coffee will cool down as its molecules transfer energy to the iceberg, causing the iceberg's molecules to speed up. The heat transfer will continue until both the coffee and the iceberg reach thermal equilibrium.
Why doesn't the iceberg warm up significantly when hot coffee is poured onto it?
-The iceberg's massive size means that it cannot be significantly warmed by the energy transferred from a single cup of coffee, as the amount of heat required to change the temperature of the iceberg is far greater than that of the coffee.
How do the physical properties of materials, like aluminum and a paperback book, affect the sensation of temperature?
-Although both objects may be at the same temperature, the aluminum feels cooler than the paperback book because it is a better conductor of heat. The aluminum conducts heat away from your skin faster, producing the sensation of cold, even though the temperature is the same.
Why does heat transfer occur faster from a human hand to aluminum than to a paperback book?
-Heat transfer occurs faster with aluminum because it is a better conductor of heat compared to the paperback book. The rate at which heat moves from your hand to the object is influenced by the material's conductivity.
How can understanding the difference between thermal energy, temperature, and heat energy improve our understanding of physical phenomena?
-By distinguishing between thermal energy, temperature, and heat energy, we can better understand how energy flows between objects and why different materials feel differently when touched. This distinction is crucial for grasping more complex concepts in physics and chemistry.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео
5.0 / 5 (0 votes)