3.3.2 - Radiação eletromagnética: Teoria Quântica - Efeito Fotoelétrico (Einstein)
Summary
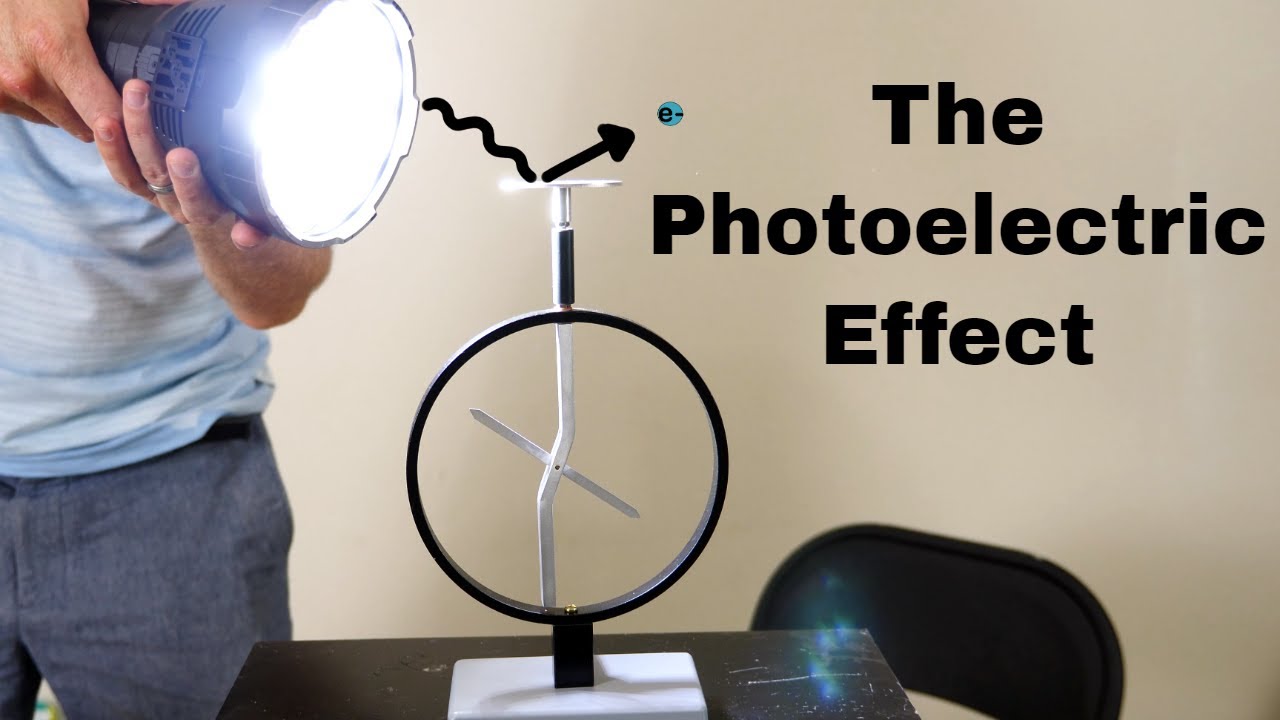
TLDRThis transcript explores the photoelectric effect, demonstrating how light can generate electric currents by ejecting electrons from a metal surface. The experiment shows that different wavelengths of light, such as red and violet, influence the ejection of electrons based on frequency and intensity. The script discusses how classical wave theory couldn't explain the results, leading Einstein to propose that light behaves both as a wave and as a particle called a photon. The energy of these photons is linked to their frequency, and this discovery played a crucial role in advancing quantum theory and earned Einstein a Nobel Prize.
Takeaways
- 😀 The photoelectric effect is the ability of electromagnetic radiation (like light) to generate electric current by ejecting electrons from a metal surface.
- 😀 The experiment involved shining light on a metal surface with two connected metal plates and observing if the light ejected electrons, thus completing an electrical circuit.
- 😀 A red light with low frequency failed to eject electrons from the metal, while violet light with higher frequency successfully ejected electrons and generated an electric current.
- 😀 Increasing the intensity of red light (more radiation) did not increase electron ejection, while increasing the intensity of violet light did, demonstrating the role of frequency in the photoelectric effect.
- 😀 The photoelectric effect cannot be fully explained by wave theory, as it depends on the frequency of the radiation, not just the amplitude.
- 😀 Amplitude, or intensity of radiation, affects the number of electrons ejected, while frequency affects the energy and speed of the ejected electrons.
- 😀 The photoelectric effect led to the conclusion that electromagnetic radiation behaves as both a wave and a particle.
- 😀 Planck's theory, associating frequency with energy, was used to explain why certain frequencies (like violet light) could eject electrons, while others (like red light) could not.
- 😀 Einstein proposed that light behaves as particles (photons) with no mass but with energy related to their frequency, providing a better explanation of the photoelectric effect.
- 😀 Not all radiation can eject electrons from a material, as sufficient energy from the photons is required to overcome the material’s work function.
- 😀 The photoelectric effect demonstrated that light, although a wave, also has particle-like properties, contributing to the dual wave-particle nature of light in quantum mechanics.
Q & A
What is the photoelectric effect?
-The photoelectric effect is the phenomenon where light, typically in the form of electromagnetic radiation, strikes a metal surface and causes the ejection of electrons, generating an electric current.
What was the initial experimental setup to observe the photoelectric effect?
-The experiment involved placing two metal plates, one connected to a battery to supply energy and the other connected to a lamp. When light was directed at the metal plate, the ejected electrons would close the circuit and light up the lamp.
What was observed when red light was used in the experiment?
-When red light, which has a low frequency, was used, no electrons were ejected from the metal surface, regardless of the light's intensity.
What change was observed when violet light was used in the experiment?
-When violet light, which has a higher frequency, was used, electrons were ejected from the metal surface. Increasing the intensity of the violet light caused the ejected electrons to gain more kinetic energy, making them ejected faster.
What did the experimenters conclude about the relationship between light's intensity and the ejection of electrons?
-The experimenters concluded that increasing the intensity of the light did not affect the energy of the ejected electrons when using red light. However, with violet light, increasing the intensity led to more electrons being ejected and increased their kinetic energy.
Why couldn't the wave theory of light explain the results of the photoelectric effect?
-The wave theory of light couldn't explain why only light above a certain frequency could eject electrons and why increasing the light's intensity did not affect the energy of the ejected electrons. It assumed that light's energy was spread out in waves, which failed to account for these observations.
How did Einstein contribute to the understanding of the photoelectric effect?
-Einstein proposed that light behaves as both a wave and a particle, called a photon. He suggested that the energy of light is quantized, meaning it depends on frequency. This explained why certain frequencies of light could eject electrons, while others could not.
What is the significance of Planck's theory in the explanation of the photoelectric effect?
-Planck's theory introduced the idea that energy is quantized and related to frequency. Einstein built on this by suggesting that light consists of photons, each carrying a specific amount of energy based on its frequency, which helped explain the photoelectric effect.
What does the concept of wave-particle duality mean in the context of light?
-Wave-particle duality refers to the concept that light can behave both as a wave and as a particle, depending on the situation. While light can diffract like a wave, the photoelectric effect shows that it can also act like a particle, with energy quantized in photons.
Why was the concept of photons important in explaining the photoelectric effect?
-The concept of photons was crucial because it explained that light consists of particles that can transfer energy to electrons. This particle-like behavior clarified why only certain frequencies of light could eject electrons and how their kinetic energy could be influenced by light's frequency.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео

Photoelectric Effect, Work Function, Threshold Frequency, Wavelength, Speed & Kinetic Energy, Electr

Dual Nature of Radiation & Matter in 10 mins 😱🔥 Ch 11 Physics Class 12 Boards 2022-23 Score 95+

¿Qué es el electromagnetismo? | 101 Videos
Knocking Electrons With Light—The Photoelectric Effect

Physics - Photoelectric effect

Física moderna: Efeito Fotoelétrico | Física
5.0 / 5 (0 votes)
