Nuclear Fission and Radioactivity - Part 1 of 3
Summary
TLDRThis video explains nuclear reactions and radioactivity, focusing on the structure of atoms and the forces that govern their stability. It discusses the role of protons, neutrons, and the strong nuclear force in keeping atomic nuclei intact. The video also explores the concept of isotopes, the line of stability, and the different types of radioactive decay: alpha, beta, and gamma emissions. Each type of decay is explained in terms of how atoms transform, with examples like uranium turning into thorium. The video concludes by emphasizing the conservation of energy, momentum, charge, and nucleon number in these processes.
Takeaways
- 😀 Atoms consist of a nucleus, which contains protons and neutrons, and electrons that orbit around it.
- 😀 The number of protons in an atom's nucleus determines the element and its position on the periodic table.
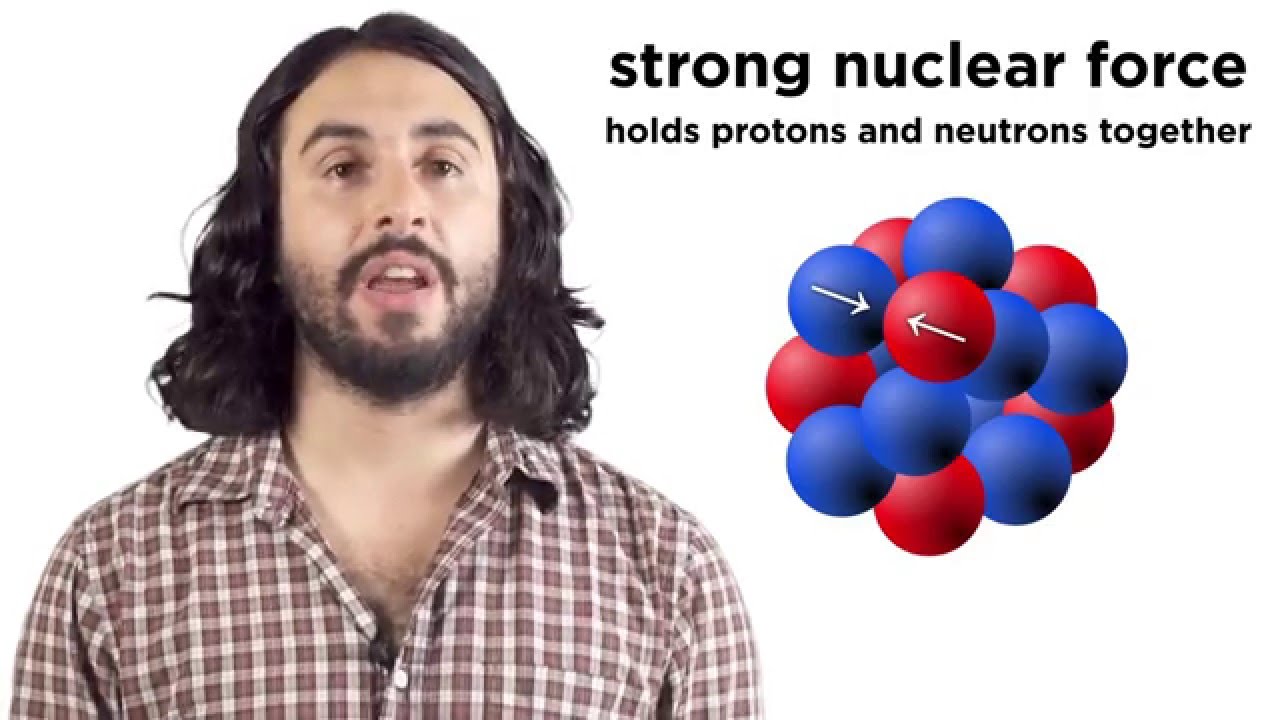
- 😀 The strong nuclear force is responsible for holding the nucleus together, overcoming the repulsion between positively charged protons.
- 😀 Atoms with approximately 100 protons in the nucleus tend to become unstable due to the balance between nuclear forces and electrostatic forces.
- 😀 The line of stability shows the relationship between protons and neutrons in stable nuclei, and elements can have various isotopes with different numbers of neutrons.
- 😀 Heavy atoms with too many protons or neutrons are likely to undergo radioactive decay to achieve stability.
- 😀 There are three main types of radioactive emissions: alpha particles, beta particles, and gamma rays.
- 😀 Alpha particles consist of 2 protons and 2 neutrons and are typically emitted by heavy elements with more than 82 protons.
- 😀 Beta particles are electrons emitted when a neutron in the nucleus decays into a proton, leading to an increase in protons but no change in nucleon number.
- 😀 Gamma rays are high-energy electromagnetic radiation emitted when a nucleus has excess energy, helping to stabilize the atom.
- 😀 During radioactive decay, energy, momentum, charge, and nucleon number are conserved, but mass is converted into energy according to Einstein’s formula E = mc².
Q & A
What is nuclear fish, and how does it relate to atomic structure?
-Nuclear fish refers to the process where a large nucleus splits into smaller nuclei. This concept is tied to nuclear reactions and radioactivity, which can occur when atoms decay by emitting alpha particles, beta particles, or gamma rays.
What is the role of protons in determining the element of an atom?
-The number of protons in an atom's nucleus determines its element. The periodic table lists elements in order of their proton number, and atoms with the same number of protons belong to the same element.
Why doesn't the nucleus of an atom break apart despite the repulsive force between protons?
-The strong nuclear force holds the nucleus together, counteracting the electrostatic repulsion between positively charged protons. This force is much stronger than the Coulomb force, especially in smaller nuclei.
What is the significance of the line of stability in the chart of isotopes?
-The line of stability represents the number of neutrons required for a stable nucleus with a given number of protons. Isotopes that deviate from this line are often unstable and may undergo radioactive decay.
How do isotopes differ from each other?
-Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This difference in neutrons can affect the stability and properties of the atom.
Why do heavier atoms tend to have more neutrons than protons?
-As elements increase in size (i.e., more protons), the strong nuclear force needs additional neutrons to help stabilize the nucleus, preventing it from breaking apart due to electrostatic repulsion between the protons.
What are the three main types of radioactive emissions?
-The three types of radioactive emissions are alpha particles (helium nuclei), beta particles (electrons), and gamma rays (high-energy electromagnetic radiation).
How does alpha decay affect the composition of a nucleus?
-During alpha decay, the nucleus loses two protons and two neutrons, effectively transforming into a new element. For example, uranium-238 undergoes alpha decay to form thorium-234.
What happens during beta decay and how does it affect the nucleus?
-In beta decay, a neutron is converted into a proton, releasing an electron (beta particle). This changes the atomic number of the element but does not affect the total number of nucleons.
What is the role of gamma rays in radioactive decay?
-Gamma rays are emitted from a nucleus that has excess energy. These high-energy electromagnetic waves carry away this energy to stabilize the nucleus, without changing the number of protons or neutrons.
What is conserved during radioactive decay, and what is not?
-During radioactive decay, energy, momentum, charge, and nucleon number are conserved. However, mass is not conserved, as some mass is converted into energy according to the equation E = mc².
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео
5.0 / 5 (0 votes)