The de Broglie Wavelength and Wave Particle Duality - A Level Physics
Summary
TLDRThis video explores the fascinating concept of wave-particle duality, which reveals that both light and particles like electrons exhibit both wave-like and particle-like behaviors depending on the context. It highlights key historical milestones, including Louis de Broglie’s 1924 theory that linked a particle's momentum to its wavelength, and the 1927 experimental confirmation through electron diffraction. The video also delves into how quantum physics emerged, blending wave and particle models to explain phenomena like diffraction and interference, while emphasizing the probabilistic nature of subatomic particles. These discoveries revolutionized our understanding of the quantum world.
Takeaways
- 😀 Wave-particle duality suggests that light and particles can exhibit both wave-like and particle-like behaviors, depending on the situation.
- 😀 De Broglie’s hypothesis (1924) proposed that particles with momentum have an associated wavelength, described by λ = h / mv.
- 😀 The relationship λ = h / mv implies that as particles move faster (with higher momentum), their associated wavelength becomes smaller.
- 😀 The wave-particle duality concept was experimentally demonstrated in 1927 when electrons showed wave-like interference patterns during diffraction experiments.
- 😀 Diffraction patterns, typically a wave phenomenon, were observed in electron experiments, indicating that electrons have wave-like properties under certain conditions.
- 😀 The intensity of a wave at a given point represents the probability of finding a particle there, underscoring the importance of probability in quantum mechanics.
- 😀 Quantum mechanics emerged as a field in the early 20th century, focusing on the dual nature of light and matter and introducing probabilistic models.
- 😀 The mathematical description of quantum phenomena, like Schrödinger’s equation, is crucial for understanding wave-particle duality and its implications.
- 😀 The wave-particle duality concept challenges traditional ideas, suggesting that particles like electrons are not just small balls, and light is not only a wave.
- 😀 Despite the challenges of visualization, quantum mechanics provides a framework for understanding behaviors that don't fit into classical wave or particle models.
Q & A
What is wave-particle duality?
-Wave-particle duality is the concept that particles, such as electrons and photons, exhibit both wave-like and particle-like properties depending on the circumstances. This duality challenges classical physics and forms a key idea in quantum mechanics.
How does the wave model explain phenomena like refraction and interference?
-The wave model explains phenomena like refraction and interference because waves can bend and overlap, creating patterns of constructive and destructive interference. These behaviors are characteristic of waves, not particles.
In what situations do we use the particle model of light?
-The particle model is used to explain phenomena like the photoelectric effect, where light interacts with matter in a way that suggests it consists of discrete particles, or photons, each carrying a specific amount of energy.
What is the significance of de Broglie's hypothesis?
-In 1924, de Broglie proposed that particles, like electrons, have a wavelength inversely proportional to their momentum. This idea linked wave-like properties to particles and became a cornerstone of quantum mechanics.
What formula did de Broglie use to describe the wavelength of a particle?
-De Broglie's formula for the wavelength of a particle is λ = h / mv, where h is Planck's constant, m is the particle's relativistic mass, and v is its velocity.
What was the significance of the 1927 experiment with electrons and diffraction?
-The 1927 experiment demonstrated that electrons, when passed through a metal, created a diffraction pattern—something typically seen with waves. This confirmed de Broglie's hypothesis and provided experimental proof of wave-particle duality.
Why do electrons form a diffraction pattern instead of traveling straight through a metal?
-Electrons form a diffraction pattern because their wavelength is comparable to the spacing between atoms in the metal. This causes the electrons to interfere with themselves, a wave phenomenon, which would not be expected from classical particles.
How did quantum physics challenge classical physics?
-Quantum physics, particularly the concept of wave-particle duality, challenged classical physics by showing that particles like light and electrons do not strictly behave as waves or particles. Instead, they exhibit properties of both, depending on the situation.
What role does probability play in quantum mechanics?
-In quantum mechanics, probability describes the likelihood of finding a particle in a specific location or state. This is a fundamental shift from classical physics, where objects are assumed to have definite positions and velocities.
Why is it difficult to imagine a particle being both a wave and a particle at the same time?
-It is difficult to imagine a particle being both a wave and a particle because our classical intuition suggests that objects must be either one or the other. However, quantum mechanics reveals that particles can exhibit both wave-like and particle-like behaviors simultaneously, which defies our everyday understanding.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео

De-Broglie Wavelength (Hypothesis) And The Wave-Like Matter

Quantum 101 Episode 1: Wave Particle Duality Explained

The biggest lie about the double slit experiment
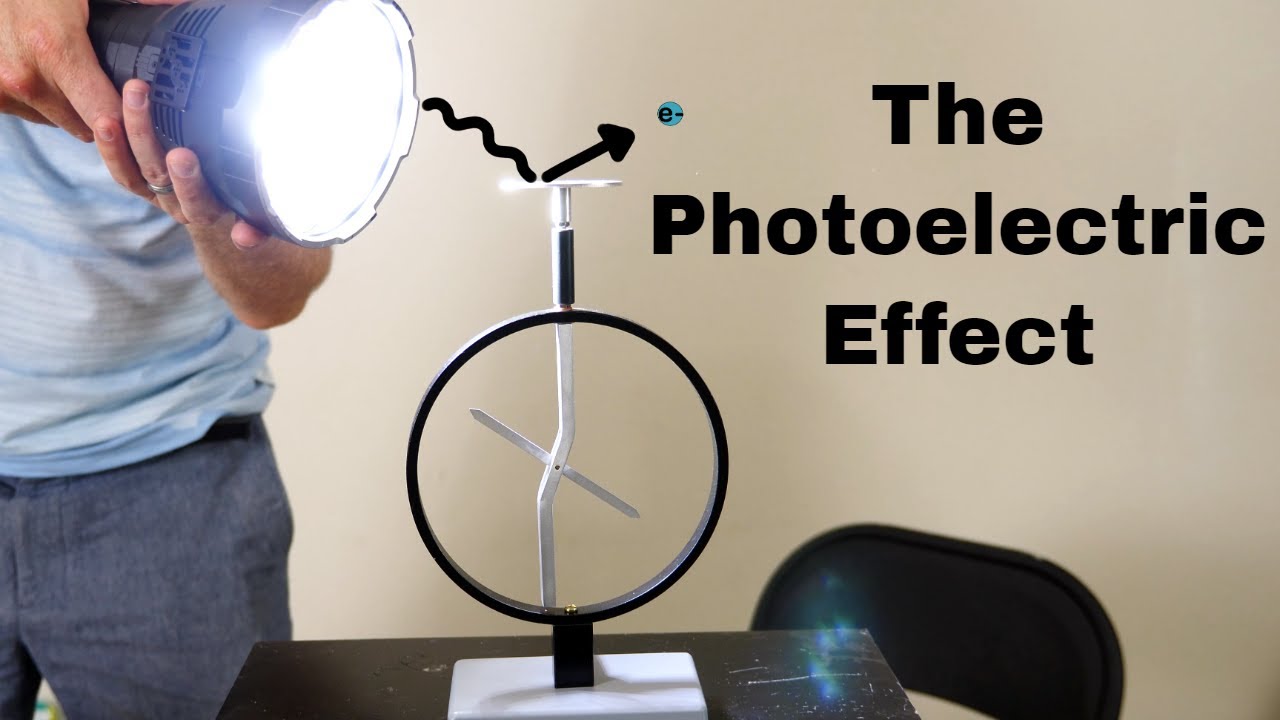
Knocking Electrons With Light—The Photoelectric Effect

The Double-Slit Experiment

Kuantum 2 .1 Hipotesis de Brogglie (Apakah Partikel Mempunyai sifat Gelombang?)
5.0 / 5 (0 votes)
