Comportement macroscopique d’un fluide
Summary
TLDRThis video capsule discusses the macroscopic behavior of fluids, focusing on the concept of fluid pressure. It explains how pressure arises from microscopic particle collisions and demonstrates this with examples, such as the pressure in a football. The relationship between pressure, force, and surface area is established, leading to the introduction of Boyle's law. This law states that at constant temperature and a fixed amount of gas, the product of pressure and volume remains constant. The video emphasizes the inverse relationship between volume and pressure and concludes with a call to remember these fundamental principles.
Takeaways
- ⚙️ Fluids exert a mechanical force on the walls they contact, modeled as a 'pressing force.'
- 📏 Pressure is defined as the force exerted by a fluid on a surface divided by the area of that surface.
- 🧮 The formula for pressure is P = F/S, where P is pressure, F is force, and S is area.
- 📊 Pressure is measured in pascals (Pa), which is equivalent to one newton per square meter.
- ⚽ An example of pressure is the higher internal pressure of a football compared to atmospheric pressure.
- 🌊 As the depth of a fluid increases, the pressure experienced by objects submerged in it also increases.
- 🔄 Boyle's Law states that at constant temperature, the product of pressure and volume for a gas remains constant (PV = constant).
- 📉 According to Boyle's Law, if the pressure of a gas increases, its volume decreases, provided the temperature is constant.
- 🎈 When a balloon is submerged deeper in water, its volume decreases due to increased pressure around it.
- 🔍 Understanding pressure, force, and volume relationships is essential in fields like engineering and fluid dynamics.
Q & A
What is the primary focus of the video transcript?
-The video discusses the macroscopic behavior of fluids, specifically focusing on fluid pressure and Boyle's law.
How is pressure defined in relation to fluids?
-Pressure is defined as the force exerted by a fluid per unit area, measured in pascals (Pa), where one pascal equals one newton per square meter.
What example is used to explain fluid pressure?
-The example used is a football, where the pressure inside the ball is greater than atmospheric pressure.
What is the relationship between pressure and surface area according to the transcript?
-The transcript states that pressure increases with a larger surface area because the force exerted on that surface is greater.
Who were the key figures mentioned in relation to Boyle's law?
-The key figures mentioned are Robert Boyle and Émile Mariotte, who independently discovered Boyle's law in the 17th century.
What does Boyle's law state?
-Boyle's law states that at constant temperature, the product of the pressure (P) and volume (V) of a gas is constant (PV = constant).
How does the volume of a gas relate to its pressure according to Boyle's law?
-According to Boyle's law, the volume of a gas is inversely proportional to its pressure when the temperature remains constant.
What happens to the volume of a balloon filled with air as it is submerged in water, based on the script?
-As the balloon descends into the water, the volume of the balloon decreases because the pressure increases with depth, in accordance with Boyle's law.
What are the units for measuring force and pressure as mentioned in the transcript?
-Force is measured in newtons (N), surface area in square meters (m²), and pressure in pascals (Pa).
What key concepts should be understood from this video?
-Viewers should understand the relationship between fluid pressure and force, as well as the application of Boyle's law regarding gas behavior under varying pressure and volume.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

Kelas XI Bab 3 Fluida Statis Part 1 Massa Jenis

Estática de Fluidos - Parte 1
Fluida Statis ( Hukum Pascal - Archimedes ) - Simple Konsep - Fisika Kelas 11
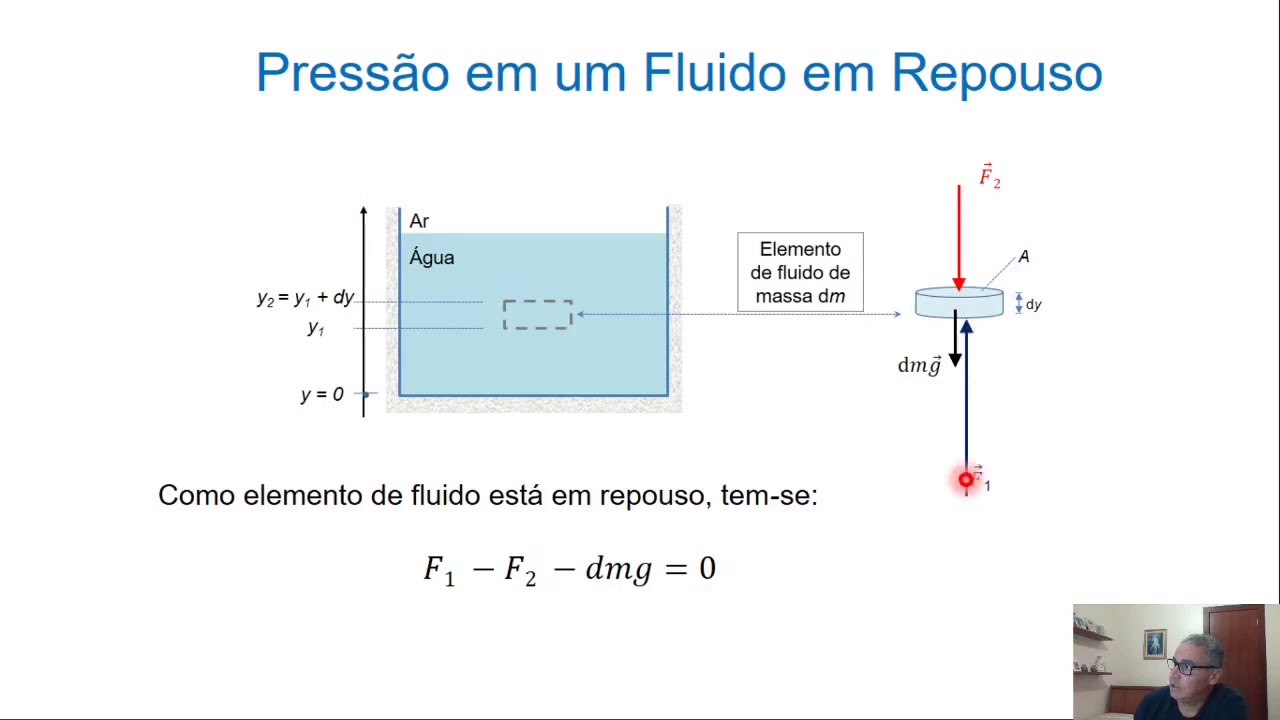
Estática de Fluidos Parte II

I fluidi, la pressione, la legge di Pascal e il torchio idraulico
Fluida Statis • Part 1: Massa Jenis, Tekanan Hidrostatis, Gaya Hidrostatis
5.0 / 5 (0 votes)
