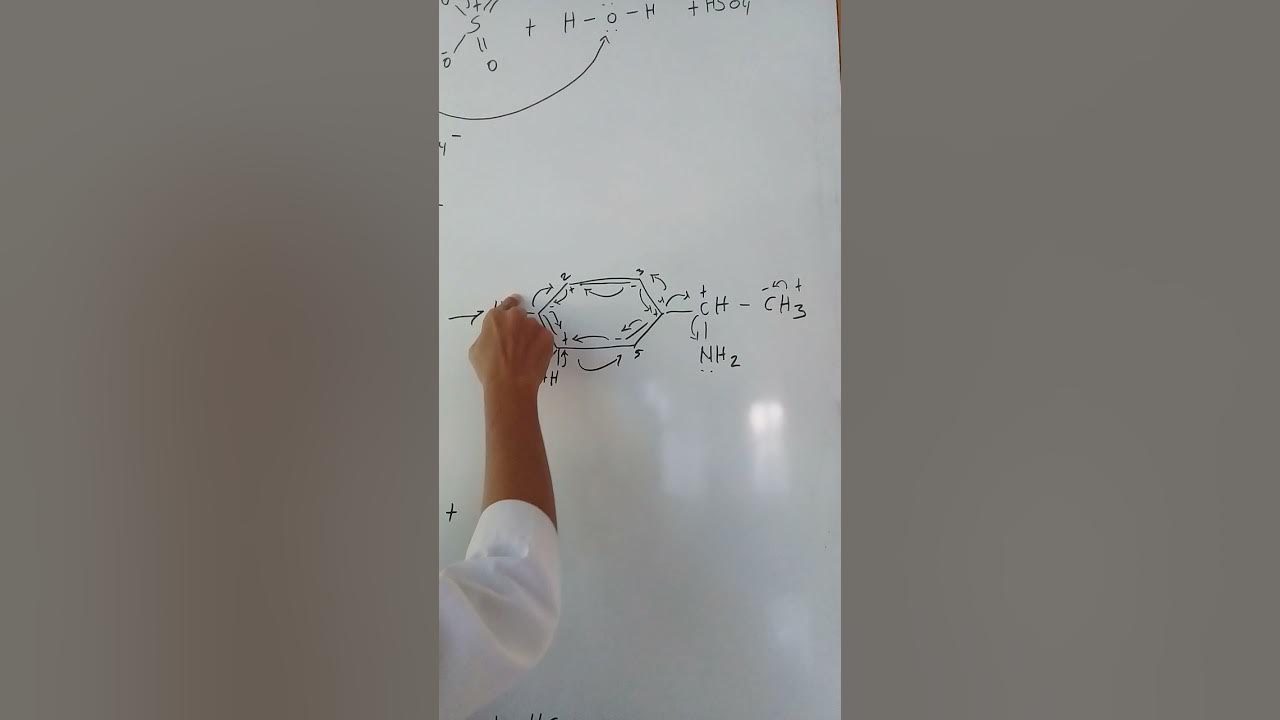
Alkene + H2SO4 + H2O
Summary
TLDRThis video explains the acid-catalyzed hydration of alkenes, focusing on the reaction between an alkene, sulfuric acid, and water. The mechanism is described in steps, starting with protonation of the alkene, followed by the formation of a carbocation intermediate, which is stabilized through hydride or methyl shifts. Water or alcohol then reacts with the carbocation to produce either an alcohol or an ether. The video also covers rearrangements like hydride and methyl shifts, and discusses ring expansions that can occur in specific cases, leading to more stable products.
Takeaways
- 🔬 The reaction between an alkene, sulfuric acid, and water produces a tertiary alcohol.
- ⚛️ The alkene acts as a nucleophile and abstracts a proton, forming a more stable carbocation intermediate.
- 💧 Water combines with the carbocation intermediate to form an oxonium species, eventually resulting in an alcohol.
- ➡️ A hydride shift occurs to stabilize the carbocation if it's adjacent to a tertiary carbon, enhancing carbocation stability.
- 🧪 Using methanol instead of water in the reaction results in the formation of an ether instead of an alcohol.
- 🔄 A methyl shift can occur when a secondary carbocation is adjacent to a quaternary carbon, enhancing the stability of the intermediate.
- 🔗 Ring expansion can take place when a secondary carbocation is near a five-membered ring, forming a more stable six-membered ring.
- ⚙️ Both hydride shifts and ring expansions increase the stability of carbocations by reducing ring strain or moving to tertiary carbons.
- 💥 The final products may have stereoisomers (R and S configurations), depending on the orientation of the nucleophilic attack.
- 🔬 The reaction mechanism differs slightly when switching solvents from water to an alcohol, resulting in different product outcomes (alcohol vs. ether).
Q & A
What happens when an alkene reacts with sulfuric acid and water?
-When an alkene reacts with sulfuric acid and water, the reaction is called acid-catalyzed hydration. The alkene first abstracts a proton from the sulfuric acid, forming a carbocation intermediate. Water then acts as a nucleophile and attacks the carbocation, leading to the formation of an alcohol, usually a tertiary alcohol if the carbocation rearranges to a more stable form.
What is the driving force for a hydride shift in the reaction mechanism?
-The driving force for a hydride shift is carbocation stability. A hydride shift occurs when a more stable carbocation can be formed, typically going from a secondary carbocation to a tertiary carbocation, which is more stable.
What type of product is formed when 3-methyl-1-butene reacts with sulfuric acid and water?
-When 3-methyl-1-butene reacts with sulfuric acid and water, the product is a tertiary alcohol. The reaction proceeds through protonation of the alkene, forming a secondary carbocation, which then undergoes a hydride shift to form a more stable tertiary carbocation, followed by nucleophilic attack by water.
How does the mechanism differ when sulfuric acid is used with methanol instead of water?
-When sulfuric acid is used with methanol instead of water, the product formed is an ether rather than an alcohol. The reaction mechanism is similar, with methanol acting as a nucleophile instead of water, ultimately leading to the formation of an ether where an oxygen atom is bonded to two carbon atoms.
What is a methyl shift, and why does it occur?
-A methyl shift is a rearrangement where a methyl group moves from one carbon to an adjacent carbon. It occurs to increase carbocation stability, typically when a secondary carbocation can shift to form a more stable tertiary carbocation.
What is the final product when 3,3-dimethyl-2-butene reacts with sulfuric acid and methanol?
-The final product when 3,3-dimethyl-2-butene reacts with sulfuric acid and methanol is an ether. The mechanism involves protonation of the alkene, followed by a methyl shift to form a more stable tertiary carbocation, and nucleophilic attack by methanol.
Why are six-membered rings more stable than five-membered rings?
-Six-membered rings are more stable than five-membered rings because they experience less ring strain. Six-membered rings have bond angles closer to the ideal tetrahedral angle of 109.5°, whereas smaller rings have more strain due to compressed angles.
What is the significance of ring expansion in carbocation rearrangements?
-Ring expansion occurs in carbocation rearrangements to reduce ring strain and increase stability. For example, a five-membered ring can expand to a six-membered ring if the formation of a more stable carbocation is possible. This makes the structure more stable overall.
What happens when both a ring expansion and hydride shift can occur in a reaction?
-When both a ring expansion and hydride shift can occur, the reaction usually undergoes both processes to maximize carbocation stability. First, the ring expansion happens to reduce strain, followed by a hydride shift to form the most stable carbocation, often a tertiary one.
Why do stereoisomers form in certain reactions involving alkene hydration?
-Stereoisomers form in reactions involving alkene hydration when the nucleophilic attack on the carbocation can occur from either side of the planar intermediate. This leads to the formation of different stereoisomers, such as R and S configurations, due to the chiral center generated in the process.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示
5.0 / 5 (0 votes)