🧪 HIBRIDAÇÃO DO CARBONO: RÁPIDO E FÁCIL
Summary
TLDRIn this lively tutorial, the host explains the concept of carbon hybridization, specifically focusing on SP3, SP2, and SP hybridizations. Using engaging analogies and humor, they describe how carbon can form four bonds, distinguishing between single, double, and triple bonds. The video emphasizes the unique characteristics of each hybridization type and encourages viewers to participate by identifying hybridization in specific carbon structures. With a mix of instruction and enthusiasm, the host aims to make complex chemistry concepts accessible and enjoyable for their audience.
Takeaways
- 😀 The video focuses on teaching carbon hybridization concepts: sp³, sp², and sp.
- 💡 Carbon can form four bonds due to its ability to hybridize.
- 🔗 sp³ hybridization occurs when carbon forms only single bonds.
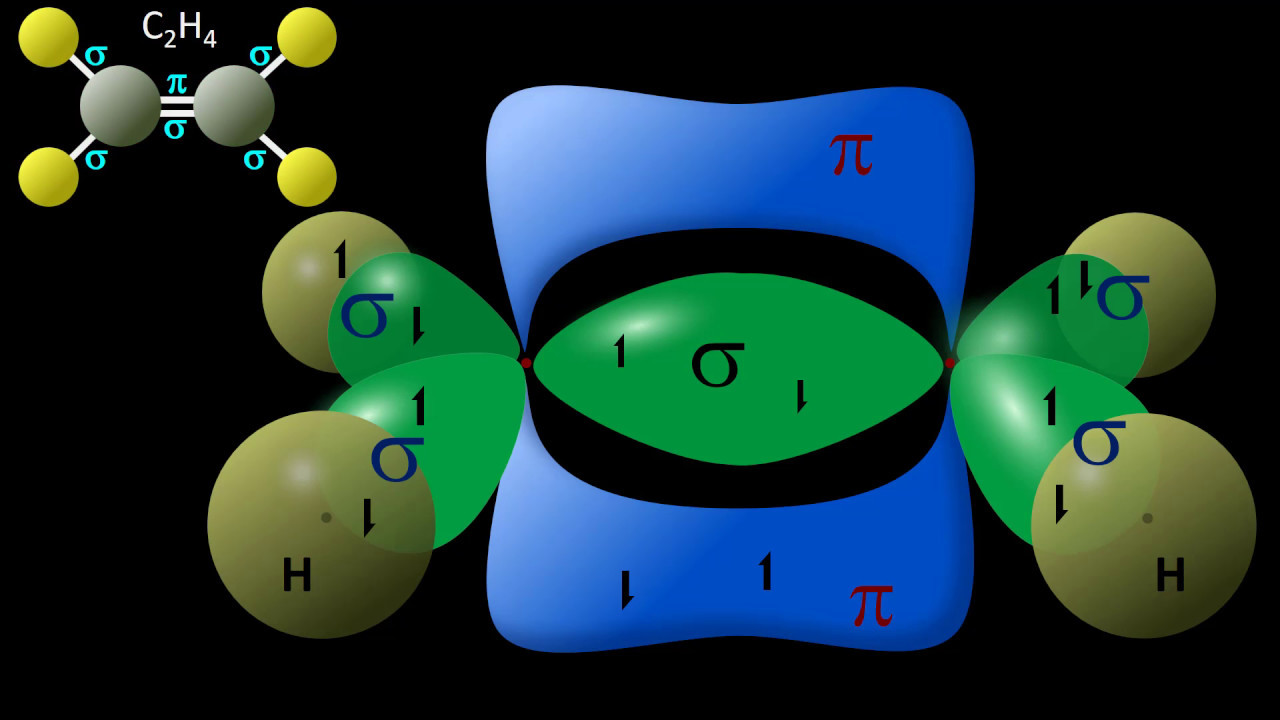
- ⚡ sp² hybridization happens when carbon forms one double bond and two single bonds.
- 🔶 sp hybridization is when carbon forms a triple bond or two double bonds.
- 🖐️ Visual representation: The speaker uses fingers to illustrate the number of bonding spaces for each hybridization type.
- 📚 The importance of understanding hybridization is emphasized for grasping carbon's bonding behavior.
- 🙌 The speaker encourages engagement by asking viewers to like and subscribe to the channel.
- 🎯 The video concludes with a challenge to determine the number of sp² carbons in a given structure.
- ❤️ The speaker expresses joy and hope that the information is helpful to viewers.
Q & A
What is hybridization in the context of carbon?
-Hybridization is a theory that explains how carbon can form four bonds by mixing its atomic orbitals to create new hybrid orbitals.
What are the three types of hybridization for carbon?
-The three types of hybridization for carbon are SP3, SP2, and SP.
When does a carbon atom undergo SP3 hybridization?
-A carbon atom undergoes SP3 hybridization when it forms four single bonds.
What does SP2 hybridization indicate about a carbon atom's bonding?
-SP2 hybridization indicates that a carbon atom forms one double bond and two single bonds.
What is the bonding characteristic of a carbon atom in SP hybridization?
-In SP hybridization, a carbon atom forms one triple bond or two double bonds.
How many bonds can a carbon atom form according to the hybridization theory?
-According to hybridization theory, a carbon atom can form four bonds.
What type of bond does hydrogen form, and how does it relate to carbon hybridization?
-Hydrogen forms only single bonds, which means that when bonded to carbon, it does not affect carbon's hybridization beyond ensuring it has one bond per hydrogen atom.
How can you determine the number of SP2 hybridized carbon atoms in a molecular structure?
-You can determine the number of SP2 hybridized carbon atoms by identifying those that form one double bond, as each of these will indicate SP2 hybridization.
What is the importance of recognizing the hybridization type in organic chemistry?
-Recognizing the hybridization type is crucial in organic chemistry because it helps predict the shape, reactivity, and properties of organic molecules.
Why is it necessary to engage with the content of the video for understanding hybridization?
-Engaging with the content is necessary because it provides practical examples and visualizations that help clarify the concept of hybridization, making it easier to understand.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen

Hybridization Theory (English)

Hybridation des orbitales atomiques (3) - Tableau récapitulatif

IR spectra for hydrocarbons | Spectroscopy | Organic chemistry | Khan Academy

MENENTUKAN BENTUK MOLEKUL : TEORI HIBRIDISASI (KIMIA SMA KELAS 10)
Hybrid Orbitals explained - Valence Bond Theory | Orbital Hybridization sp3 sp2 sp

Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3
5.0 / 5 (0 votes)
