Kalor - Fisika Kelas X
Summary
TLDRThis video script provides an in-depth explanation of fundamental thermodynamic concepts, such as heat transfer, specific heat capacity, latent heat, and phase changes. It highlights how heat moves between substances, the concept of thermal equilibrium, and the formulas used to calculate changes in temperature and energy. The script includes practical examples, including calculations for heating water and evaporating liquids, and discusses the relationship between different units of heat. Additionally, it covers energy conservation, phase changes like melting and boiling, and uses real-life scenarios to demonstrate how heat transfer works in various systems.
Takeaways
- 😀 Heat transfer occurs when two liquids with different temperatures are mixed, and they reach an equilibrium temperature between the two initial temperatures.
- 😀 Calorimetry defines heat as the transfer of energy from one system to another, typically associated with temperature change.
- 😀 The SI unit of heat is Joule, and one calorie equals 4.18 Joules.
- 😀 Different substances have different specific heat capacities, which define their ability to absorb heat. Higher specific heat means higher ability to absorb heat.
- 😀 Specific heat is mathematically expressed as C, where Q is heat, m is mass, and ΔT is the change in temperature.
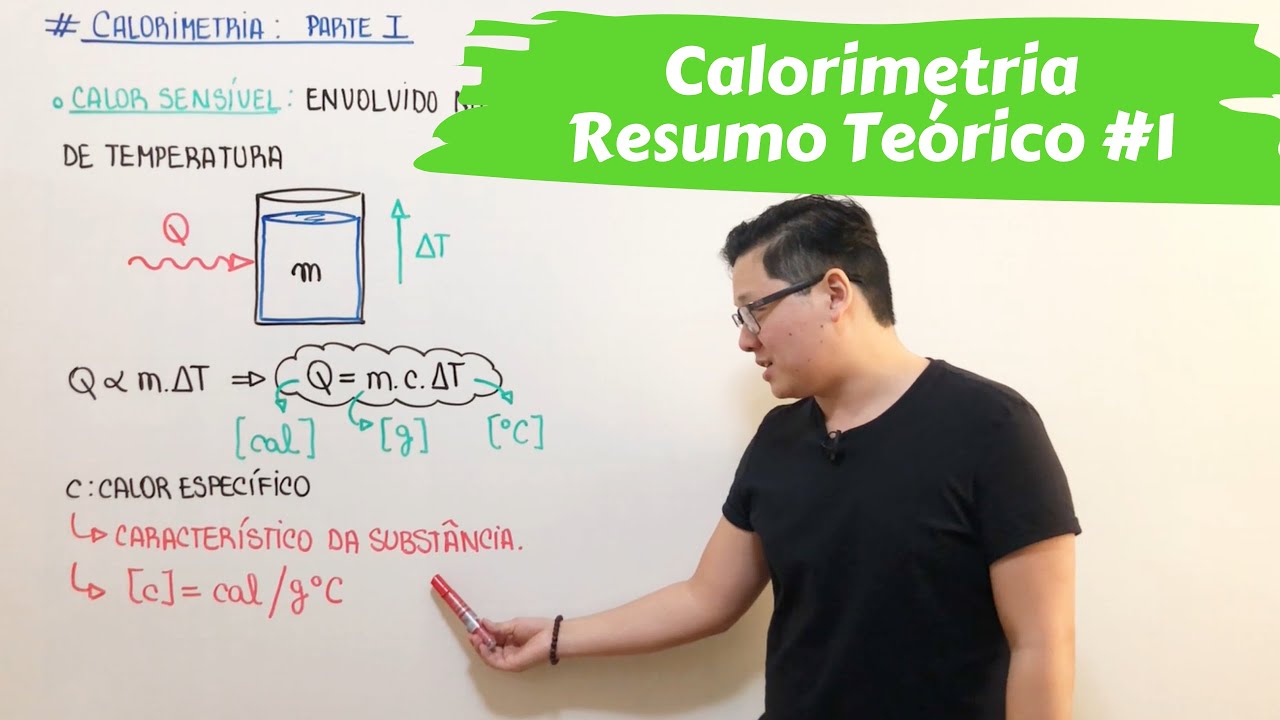
- 😀 The heat capacity of a substance is the product of specific heat and mass, represented as C = m × Cₚ.
- 😀 Heat required to change the temperature of a substance can be calculated using the formula Q = m × C × ΔT.
- 😀 Latent heat is the energy required for a substance to change its phase, such as from solid to liquid or liquid to gas, without a change in temperature.
- 😀 The latent heat of vaporization is the heat required to turn a liquid into a gas, while the latent heat of fusion is the heat needed to melt a solid.
- 😀 When a substance undergoes a phase change, the temperature remains constant, but heat is still transferred to overcome molecular forces.
- 😀 The principle of heat exchange states that the heat lost by a hotter substance equals the heat gained by a cooler substance in a closed system.
Q & A
What happens when two liquids at different temperatures are mixed together?
-When two liquids at different temperatures are mixed, they will eventually reach an equilibrium temperature that lies between the initial temperatures of the liquids. Heat flows from the liquid with the higher temperature to the one with the lower temperature until both liquids are at the same temperature.
What is heat and how is it defined?
-Heat is defined as the transfer of energy from one object to another due to a temperature difference. It results in a change in temperature and is measured in joules (J) in the SI unit system, with one calorie equivalent to 4.18 joules.
Why do two objects made of different materials, like aluminum and copper, heat up differently when exposed to the same conditions?
-Different materials have different specific heat capacities. Specific heat capacity is the amount of heat needed to raise the temperature of a substance by 1 degree Celsius. Materials with a higher specific heat capacity require more heat to change their temperature.
What is specific heat capacity and how is it calculated?
-Specific heat capacity is the amount of heat required to raise the temperature of a substance by 1 degree Celsius. It is calculated using the formula Q = m × c × ΔT, where Q is the heat absorbed, m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
How do you calculate the final temperature of water after heating?
-To calculate the final temperature of water after heating, use the formula Q = m × c × ΔT, where Q is the heat added, m is the mass, c is the specific heat capacity of water, and ΔT is the change in temperature. Rearranging the formula gives the final temperature.
What are the three states of matter and how does heat influence them?
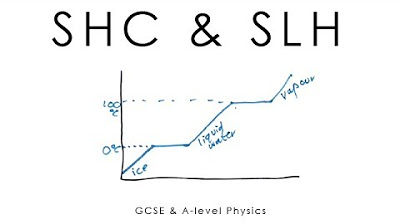
-The three states of matter are solid, liquid, and gas. Heat affects these states by causing substances to melt (solid to liquid), vaporize (liquid to gas), or condense (gas to liquid). Changes in state involve the absorption or release of heat, but the temperature remains constant during the phase transition.
What is latent heat and how is it related to phase transitions?
-Latent heat is the heat required to change the phase of a substance without changing its temperature. It can be classified into latent heat of fusion (for melting) and latent heat of vaporization (for vaporization). The amount of heat required for a phase transition is proportional to the mass of the substance.
How do you calculate the heat required for the evaporation of water?
-The heat required for evaporation is calculated using the formula Q = m × L, where Q is the heat required, m is the mass of the substance, and L is the latent heat of vaporization. For water, the latent heat of vaporization is 2,200,000 joules per kilogram.
What is the significance of latent heat in phase changes?
-Latent heat plays a crucial role during phase changes as it is the energy required to overcome the forces holding the molecules together. During melting or vaporization, heat energy is used to break molecular bonds, and temperature remains constant until the phase transition is complete.
What does the principle of heat exchange state?
-The principle of heat exchange states that when two bodies at different temperatures come into contact, the amount of heat lost by the hotter object equals the amount of heat gained by the cooler object, until both objects reach the same temperature. This is based on the law of conservation of energy.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
Specific Heat Capacity + Latent Heat - GCSE & A-level Physics (full version)
Termologia | Calorimetria - Parte I (RESUMÃO)

Hukum Termodinamika, Bagian 2: Entalpi

KELAS FISIKA_KALOR_PART2

KALOR DAN PERPINDAHAN KALOR SERTA CONTOH SOAL DAN PENYELESAIANNYA|| Kelas XI SMA || Gasal

Termologia | Calorimetria - Parte II (RESUMÃO)
5.0 / 5 (0 votes)
